 Funding/financial disclosures. The author has no conflicts of interest relevant to the content of this letter. No funding was received for the preparation of this letter.
Funding/financial disclosures. The author has no conflicts of interest relevant to the content of this letter. No funding was received for the preparation of this letter.
Innov Clin Neurosci. 2022;19(10–12):8–9.
Dear Editor:
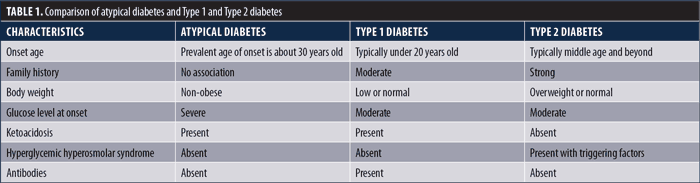
Patients exposed to second-generation antipsychotics (SGAs) are at approximately 10 times greater risk of developing diabetic ketoacidosis (DKA), compared to the general population.1 The majority of patients who develop DKA following treatment with antipsychotics are treated with olanzapine or clozapine.2 DKA more often develops in patients with Type 1 diabetes (T1DM), but DKA caused by olanzapine often lacks autoantibodies detected in T1DM, including autoantibodies against glutamic acid decarboxylase (GAD), insulinoma antigen-2 (IA-2A), insulin (IAA), and zinc transporter 8 (ZnT8A). According to the American Diabetes Association (ADA), diabetes can be broadly classified into four clinical categories: T1DM, Type 2 diabetes (T2DM), gestational diabetes, and atypical diabetes.3 Features of atypical diabetes include development around the age of 30 years, lack of obesity, no family history of diabetes, no autoantibodies, presence of hyperglycemia, and higher risk of DKA at onset of diabetes that improves with a period of insulin therapy.4 Atypical forms of diabetes often fall into this category. Olanzapine-induced DKA develops from atypical diabetes, not T1DM or T2DM, and the mechanisms are not well understood (Table 1).
Metabolic disturbances caused by SGAs such as olanzapine were traditionally thought to be related to the receptor profiles of antipsychotics, not the mechanisms that induce atypical diabetes. At the cellular level, olanzapine induces endoplasmic reticulum (ER) stress in various cells. Mechanisms of obesity and T2DM also involve ER stress induced by olanzapine. Olanzapine significantly activates protein kinase R-like endoplasmic reticulum kinase (PERK) in human neuroblastoma-derived cells in a dose-dependent manner, suggesting that it induces ER stress.5 Olanzapine treatment for eight days in rats was also observed to activate PERK and its downstream eukaryotic initiation factor 2α (eIF2α) in the hypothalamus, accompanied by increased food intake and weight gain. Co-treatment with the ER stress inhibitor, 4-phenylbutyrate, dose-dependently inhibited olanzapine-induced activated PERK-eIF2α.5 Olanzapine causes ER stress in the hypothalamus, which increases appetite and leads to weight gain. Although this mechanism is consistent with the development of T2DM, it does not explain the development of DKA.
The pathogenesis of atypical diabetes caused by olanzapine is a reversible, temporary cessation of insulin secretion that is not mediated by immunologic effects on beta cells.6 We investigated the possibility that olanzapine induces ER stress in pancreatic beta cells in cell lines and mice. Olanzapine induced ER stress by activating PERK-eIF2α signaling in hamster beta cells. In olanzapine-treated beta cells, phosphorylation of eIF2α, which reduces ER stress, did not occur, resulting in continued ER stress and decreased insulin secretion.7 Further treatment of another beta-cell line, INS-1 cells, with olanzapine increased PERK-eIF2α, inositol-requiring enzyme 1 (IRE-1) phosphorylation, and X-box binding protein 1 (XBP1) splicing and activated ER stress-mediated signaling.8 To explore the mechanism by which olanzapine induces ER stress in beta cells and decreases insulin secretion, we used mouse insulinoma MIN6 cells. Olanzapine inhibited the proper disulfide bonding of proinsulin, resulting in polymerization of proinsulin, decreased maturation of proinsulin, and inhibited proinsulin secretion.9 This mechanism of olanzapine-induced beta cell dysfunction could explain metabolic disturbances not associated with weight gain and insulin resistance. Epidemiological studies have shown that DKA occurs more frequently during the first six months of olanzapine administration, which also indicates that DKA does not develop with weight gain or insulin resistance.1 Early activation of ER stress in pancreatic beta cells is a potential mechanism behind olanzapine-induced changes in glucose homeostasis. ER stress might be a contributing factor to olanzapine-induced atypical diabetes in which insulin secretion stops, as in T1DM.
Olanzapine induces ER stress in a variety of cells. A recent report showed that olanzapine induced ER stress in the rat frontal lobe and activated the immune system.10 Olanzapine also induces ER stress in neurons, which affects mental function via immune regulation. Further studies focusing on ER stress induced by olanzapine are needed to elucidate the mechanisms behind these side effects of olanzapine. Studies of protein synthesis in cells might be useful to fill in the limitations of studies based on receptor profiles to elucidate the effects of SGAs on the human body.
References
- Polcwiartek C, Vang T, Bruhn CH, et al. Diabetic ketoacidosis in patients exposed to antipsychotics: a systematic literature review and analysis of Danish adverse drug event reports. Psychopharmacology (Berl). 2016;233(21–22):3663–3672.
- Vuk A, Kuzman MR, Baretic M, Osvatic MM. Diabetic ketoacidosis associated with antipsychotic drugs: case reports and a review of literature. Psychiatr Danub. 2017;29(2):121–135.
- American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012 ;35(Suppl 1):S11–S63.
- Nagamine T. Atypical diabetes mellitus caused by olanzapine. Aust N Z J Psychiatry. 2021;55(12):1207–1208.
- He M, Huang XF, Gao G, et al. Olanzapine-induced endoplasmic reticulum stress and inflammation in the hypothalamus were inhibited by an ER stress inhibitor 4-phenylbutyrate. Psychoneuroendocrinology. 2019;104:286–299.
- Nagamine T. Olanzapine and diabetic ketoacidosis: what is the underlying mechanism? Innov Clin Neurosci. 2018;15(3–4):11.
- Ozasa R, Okada T, Nadanaka S, et al. The antipsychotic olanzapine induces apoptosis in insulin-secreting pancreatic β cells by blocking PERK-mediated translational attenuation. Cell Struct Funct. 2013;38(2):183–195.
- Grajales D, Vázquez P, Alén R, et al. Attenuation of olanzapine-induced endoplasmic reticulum stress improves insulin secretion in pancreatic beta cells. Metabolites. 2022;12(5):443.
- Ninagawa S, Tada S, Okumura M, et al. Antipsychotic olanzapine-induced misfolding of proinsulin in the endoplasmic reticulum accounts for atypical development of diabetes. Elife. 2020;9:e60970.
- Li WT, Huang XF, Deng C, et al. Olanzapine induces inflammation and immune response via activating ER stress in the rat prefrontal cortex. Curr Med Sci. 2021;41(4):788–802.
With regards,
Takahiko Nagamine, MD, PhD
Dr. Nagamine is with the Department of Emergency Medicine and Psychiatric Internal Medicine at Sunlight Brain Research Center in Yamaguchi, Japan.


