
by Shemonti Hasan, MD; Charles H. Adler, MD, PhD; Nan Zhang, MS;
Geidy E. Serrano, PhD; Lucia I. Sue, BS; Holly A. Shill, MD; Shyamal H. Mehta, MD, PhD; Thomas G. Beach, MD, PhD; and Erika D. Driver-Dunckley, MD
Drs. Hasan, Adler, Mehta, and Driver-Dunckley are with the Parkinson’s Disease and Movement Disorders Center, Department of Neurology, Mayo Clinic in Scottsdale, Arizona. Ms. Zhang is with the Department of Health Science Research, Section of Biostatistics, Mayo Clinic in Scottsdale, Arizona. Drs. Serrano and Beach and Ms. Sue are with Civin Laboratory for Neuropathology, Banner Sun Health Research Institute in Sun City, Arizona. Dr. Shill is with Barrow Neurological Institute in Phoenix, Arizona.
Funding: The Brain and Body Donation Program has been supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
Disclosures: Dr. Adler has received funding from the Michael J. Fox Foundation and consulting fees from Jazz, Neurocrine, Scion, and Sunovion. Dr. Shill has received research support from Biogen; Dong-A ST Co., Ltd.; MagQu. Intec Pharma, Ltd.; US World Meds; and Sunovion/Cynapsus Therapeutics, Inc. and consulting honoraria for advisory boards from AbbVie and Sunovion. Dr. Beach has received research funding from the National Institutes of Health (P30 AG19610), Michael J. Fox Foundation for Parkinson’s Research, Department of Health and Human Services of the State of Arizona, Avid Radiopharmaceuticals, Navidea Biopharmaceuticals, and Aprionoia Therapeutics. Dr. Driver-Dunckley has received research support from Biogen. All other authors have no conflicts of interest relevant to the contents of this article.
Innov Clin Neurosci. 2022;19(10–12):19–23.
Abstract
Objective: Measuring olfactory dysfunction shows promise as one of a number of nonmotor biomarkers that can be used to detect clinically manifest and prodromal Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) and to differentiate these from nonsynucleinopathies. Using a larger sample size than in our previous study, we evaluated the relationship between olfactory dysfunction based on the University of Pennsylvania Smell Identification Test (UPSIT) to the clinicopathological findings in patients with PD (n=41), patients with incidental Lewy body disease (ILBD) (n=47), and controls with no neurodegenerative disease (n=137).
Design: This study was conducted through the Arizona Study of Aging and Neurodegenerative Disease (AZSAND). We selected individuals who had an UPSIT score completed antemortem and were clinicopathologically diagnosed with PD, ILBD, or control. Various measures included density of Lewy type synucleinopathy (aSyn) in the olfactory bulb and tract, as well as connected mesial temporal lobe structures. Cases and controls were analyzed using one-way analysis of variance (ANOVA) with pairwise contrasts.
Results: Compared to controls (mean: 27.8, standard deviation [SD]: 6.0), the mean UPSIT scores were lower for PD (15.8, SD: 6.0, p<0.001) and ILBD (24.1, SD: 8.6, p<0.001). The sensitivity for detecting ILBD from controls, based on a cutoff score of less than 23 (23/47), was 48.9 percent. The specificity for detecting a control was 79.6 percent with a cutoff greater than 23 (109/137).
Conclusion: These findings replicate, with a larger sample size, our previously published findings that individuals with autopsy-confirmed PD and ILBD have significantly lower UPSIT scores compared to controls. These data add to the growing body of evidence supporting early olfactory dysfunction as a prodromal biomarker for the risk of developing PD and ILBD as a prodromal Lewy body disorder.
Keywords: Hyposmia, Parkinson’s disease, incidental Lewy body disease
Identifying nonmotor biomarkers for Parkinson’s disease (PD) and dementia with Lewy body disease (DLB) presents an opportunity to detect the disease prior to widespread central nervous system (CNS) spread of Lewy type synucleinopathy (aSyn), with consequent severe dopaminergic neuronal loss in the substantia nigra and progression to motor and cognitive dysfunction. Some of these nonmotor biomarkers include olfactory dysfunction, visual changes, and constipation.1 Among patients with rapid eye movement (REM) sleep behavior disorder (RBD) who eventually developed PD, recent data demonstrated that olfactory dysfunction can present 20 years or more prior to motor dysfunction and PD diagnosis.2,3 Olfactory loss is associated with higher relative risk of PD in men, compared to women, and in White individuals, compared to Black individuals.4 Olfactory dysfunction correlates with neuropathological findings in autopsied PD and DLB brains demonstrating severe aSyn in the olfactory bulb5 and might help differentiate PD and DLB from nonsynucleinopathies, such as progressive supranuclear palsy.6–8 Detecting olfactory dysfunction early could be useful, as it can be easily and objectively measured using tests such as the University of Pennsylvania Smell Identification Test (UPSIT).9
In addition to PD, olfactory dysfunction is observed in incidental Lewy body disease (ILBD).10 The presence of Lewy bodies in autopsied brains of individuals with no history of parkinsonism or dementia is classified as ILBD. ILBD cases also exhibit significantly decreased striatal tyrosine hydroxylase, the principal enzyme for dopamine synthesis, compared to controls with no neurodegenerative disease.11–13 It is therefore thought that ILBD might represent prodromal PD or DLB. Discovering ways to detect ILBD presents an opportunity to detect synucleinopathies before progression toward motor dysfunction or dementia.
Our group previously studied olfactory dysfunction in putatively prodromal synucleinopathies by comparing UPSIT scores between patients with clinicopathologically diagnosed PD, those with ILBD, and controls with no neurodegenerative disease.14 We identified significantly lower UPSIT scores among PD and ILBD cases, compared to controls, suggesting that olfactory testing could be useful for identifying prodromal PD.14 The objective of this study was to reevaluate and confirm these findings with a larger cohort.
Methods
Patients. This study was performed as part of the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND) through the Arizona Parkinson Disease Consortium/Banner Sun Health Research Institute Brain and Body Donation Program (BBDP). All participants signed an informed consent form to undergo yearly standardized medical, movement, and cognitive assessments while alive and donation of their body and brain to the program.15,16 The study was approved by Banner Sun Health Research Institute’s designated Institutional Review Board (IRB).
Diagnosis of PD and ILBD. Cases with PD or ILBD were included in the analysis. These cases were diagnosed based on clinical-pathological correlation after autopsy, as previously described;15,16 ILBD cases were defined as having aSyn but not parkinsonism or dementia. Controls were cases without a neurodegenerative disease. The following clinical-pathological diagnoses were excluded: Alzheimer’s disease, vascular dementia, dementia not otherwise specified (NOS), dementia with Lewy bodies, PD with dementia, other forms of parkinsonism, progressive supranuclear palsy, multiple system atrophy, cerebellar degeneration, amyotrophic lateral sclerosis, meningioma, multiple sclerosis, glioblastoma, radiation necrosis, and brain cancer. Individuals with a final cognitive diagnosis of mild cognitive impairment or any form of cognitive impairment without dementia were not excluded from any group.
UPSIT. The UPSIT is an olfactory test where the participant identifies 40 odorants microencapsulated on “scratch and sniff” labels.9 The user identifies the correct scent through multiple choice questions. The UPSIT is administered by trained technicians and scored using standard procedures (score 0–40), with a higher score indicating stronger olfaction. This test can be done in 10 to 15 minutes, making it a time- and cost-efficient self-report tool. The raw score was calculated as the number of correctly identified odors.9 The last UPSIT score recorded during life was used for analysis.
aSyn densities. For all subjects, aSyn densities were recorded from 10 separate brain regions, including the olfactory bulb and tract, as well as closely connected mesial temporal lobe brain regions (amygdala, transentorhinal area), and cases were classified by the Unified Staging System for Lewy Body Disorders (USSLB).17 Densities were graded with a five-point semiquantitative scale using formalin-fixed, paraffin-embedded 5μm sections immunohistochemically stained using an antibody against phosphorylated alpha-synuclein peptide (1:10,000; rabbit polyclonal antihuman phosphoserine 129).18
Statistical analysis. Analysis of variance (ANOVA) test, Wilcoxon rank sum test, or Chi-squared test were used as appropriate to compare the differences in demographics and clinical characteristics among the three groups. Further ANOVA tests and linear models adjusting for confounding variables (sex, smoking history, and age) were used to investigate if there were differences in UPSIT scores among the three groups. Additionally, pairwise comparisons were implemented where warranted. The Bonferroni method was used to adjust for multiple comparisons. Receiver-operating characteristics (ROC) analysis was implemented together with Youden index to choose an optimum UPSIT cut-off point to differentiate ILBD from control patients. Sensitivity, specificity, accuracy, and area under the ROC curve were calculated. SAS software (SAS Institute, 2012, v9.3) was used for analysis and statistical significance was defined at p less than 0.05.
Results
Of the 902 cases in the BBDP, 332 met study criteria, of whom 225 in the targeted diagnostic groups had completed at least one UPSIT test (PD: n=41, ILBD: n=47, controls: n=137). (Figure 1).
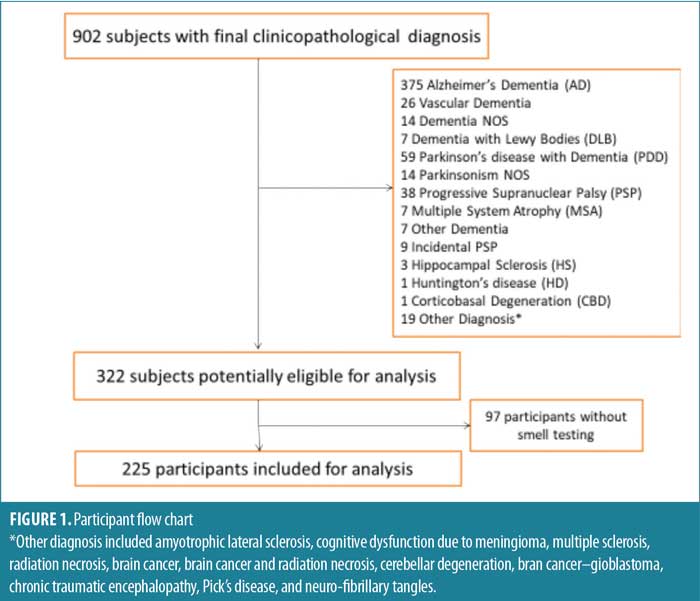
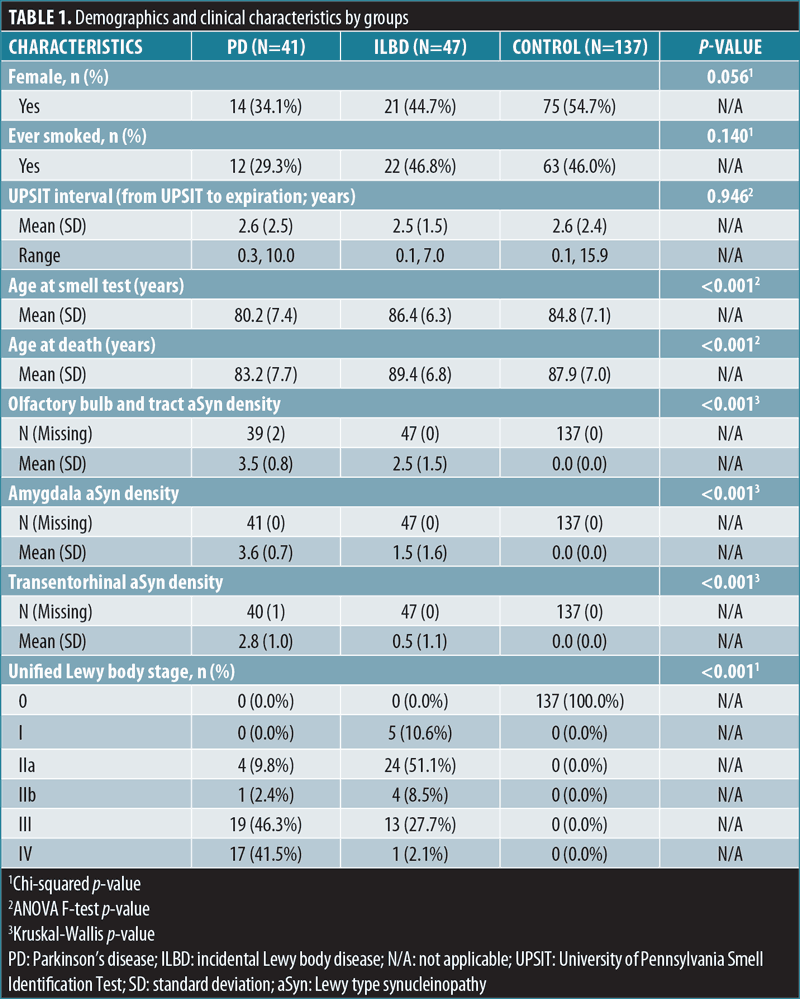
Table 1 displays the demographic and clinical characteristics among the cases and controls. On average, PD cases were younger at the time of death (83.2 years) compared to ILBD (89.4 years) and controls (87.9 years) (p≤0.001). The mean age when the smell test was administered was highest for patients with ILBD (86.4 years), followed by controls (84.8 years) and patients with PD (80.2 years) (p≤0.001). The mean interval between the last UPSIT test and death among all the subjects was 2.6 years, with no statistically significant difference between each group. There was no significant difference in sex or smoking history between the groups.
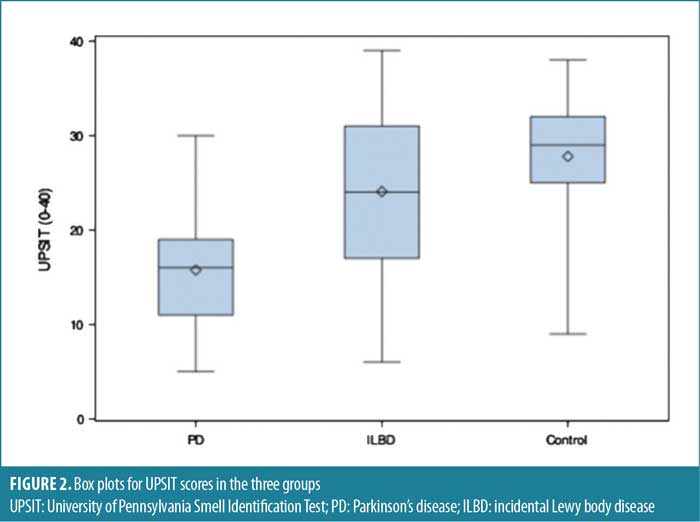
When comparing the UPSIT scores among the groups, PD cases had the lowest scores (mean: 15.8, standard deviation [SD]: 6.0), followed by ILBD (mean: 24.1, SD: 8.6) and controls (mean: 27.8, SD: 6.0). The differences between these groups were statistically significant, even with pairwise comparisons using the Bonferroni method to adjust for multiple comparisons. Multivariable linear regression was used to adjust for age at smell test, sex, and smoking history (Table 2). After adjusting, the observed overall and pairwise differences among the groups remained significant. Figure 2 shows the score differences between the three groups.

When comparing the aSyn densities across the brain regions investigated here (0–4), PD cases had the highest densities in the olfactory bulb and tract (mean: 3.5, SD: 0.8), followed by ILBD (mean: 2.5, SD: 1.5) and controls (mean: 0.0, SD: 0.0; p≤0.001). This order was also found for aSyn scores in the amygdala and transentorhinal cortex (Table 1). Among the ILBD cases, five (10.6%) were USSLB Stage I, 24 (51.1%) were Stage IIa, four (8.5%) were Stage IIb, 13 (27.7%) were Stage III, and one (2.1%) was Stage IV. In contrast, among the PD cases, zero were Stage I, four (9.8%) were Stage IIa, one (2.4%) was Stage IIb, 19 (46.3%) were Stage III, and 17 (41.5%) were Stage IV (Table 1).
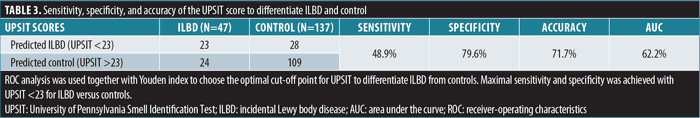
ROC analysis was performed using the Youden index to choose an optimal UPSIT cut-off point for differentiating between controls and ILBD cases. An UPSIT score of 23 was chosen as the optimal cut-off, with a sensitivity of 48.9 percent, specificity of 79.6 percent, and accuracy 71.7 percent (Table 3). The positive likelihood ratio of hyposmia in ILBD was 2.4, while the negative likelihood ratio was 0.6.
Discussion
Clinicopathologically diagnosed ILBD and PD cases had significantly lower UPSIT scores, compared to controls, both in the unadjusted and adjusted models. Our findings from this study, with larger group sizes, confirm our previous findings.14 Our current study included 225 subjects (41 with PD, 47 with ILBD, 137 controls), whereas our previous study included 92 subjects (10 with PD, 13 with ILBD, 69 controls).
If ILBD is indeed a precursor to PD, this would explain why the UPSIT scores for patients with ILBD were significantly lower than the scores of the controls, but higher than those of patients with PD. Our findings agree with other studies that demonstrated aSyn in the olfactory bulb is sensitive and specific for Lewy body disorders,4 and olfactory dysfunction is associated with PD, DLB, and ILBD.1,6–8,10,19 Our findings also correlate with other studies, indicating that patients with hyposmia have a higher likelihood ratio of having PD than those without hyposmia.20 Together with previously reported studies showing decreased striatal tyrosine hydroxylase in ILBD, these findings support the hypothesis that ILBD represents prodromal Lewy body disease.11,12
Identification of reliable markers requires prospective studies. Studies in high-risk populations are susceptible to selection bias and limited generalizability. Self-reported olfactory loss is also sensitive but not specific for PD because it can confound with cognitive impairment and depression.21 Our study accounted for these issues by recording serial UPSIT scores prior to diagnosis, which was based on clinical-pathological correlation after autopsy. In this postmortem pathology-anchored study, we also accounted for comorbid pathologies using multivariable statistics.
The discovery of biomarkers for PD is crucial since there is an upward of 60-percent loss of dopaminergic neurons in the nigrostriatal pathway prior to motor onset.1 Studies describing the staging of Lewy body disorders demonstrate that Lewy bodies can be present in other areas of the brain, such as the medulla and olfactory bulb, without affecting the substantia nigra.9,22 Lewy bodies have also been found in the spinal cord, vagus nerve, submandibular gland, and lower esophagus, which could explain other nonmotor symptoms associated with PD.23 These findings highlight the potential usefulness of nonmotor PD symptoms for identifying prodromal or premotor PD. Performing smell tests to detect olfactory impairment in at-risk individuals (e.g., RBD; genetic predisposition; relatives of PD patients hyposmia; individuals with other nonmotor prodromal symptoms, such as orthostatic hypotension, erectile dysfunction, etc.) would be helpful since nearly 70 percent of patients with PD might be unaware of their impairment.24,25 Since olfactory dysfunction can present years prior to the onset of motor symptoms in PD, this would provide physicians a window of opportunity to intervene with a potential disease-modifying therapy.
Limitations and Conclusion
As the largest cohort of ILBD cases to date, our study confirmed that ILBD is associated with hyposmia. The specificity of the finding is close to 80 percent, meaning that false positives might be as low as 20 percent. However, the sensitivity is less useful, at only about 50 percent. When screening otherwise normal subjects for prodromal Lewy body disease, olfactory testing might best be combined with other biomarkers.
Ethics Statement
The Banner Sun Health Research Institute IRB and Mayo Clinic IRB approved this study. All subjects signed informed consent and were enrolled in the AZSAND, an ongoing longitudinal clinicopathologic study in the Banner Sun Health Research Institute BBDP.
Acknowledgments
AZSAND and the BBDP are supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium), the Michael J. Fox Foundation for Parkinson’s Research, and Mayo Clinic Foundation.
References
- Adler CH. Premotor symptoms and early diagnosis of Parkinson’s disease. Int J Neurosci. 2011;121(Suppl 2):3–8.
- Boeve BF. REM sleep behavior disorder: updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. 2010;1184:15–54.
- Fereshtehnejad SM, Yao C, Pelletier A, et al. Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: a prospective study. Brain. 2019;142(7):2051–2067.
- Chen H, Shrestha S, Huang X, et al. Olfaction and incident Parkinson disease in US white and black older adults. Neurology. 2017;89(14):1441–1447.
- Beach TG, White CL, Hladik CL, et al. Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol. 2009;117(2):169–174.
- Beach TG, Adler CH, Zhang N, et al. Severe hyposmia distinguishes neuropathologically confirmed dementia with Lewy bodies from Alzheimer’s disease dementia. PloS One. 2020;15(4):e0231720.
- Gerkin RC, Adler CH, Hentz JG, et al. Improved diagnosis of Parkinson’s disease from a detailed olfactory phenotype. Ann Clin Transl Neurol. 2017;4(10):714–721.
- Shill HA, Zhang N, Driver-Dunckley E, et al. Olfaction in neuropathologically defined progressive supranuclear palsy. Mov Disord. 2021;36(7):1700–1704.
- Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489–502.
- Ross GW, Abbott RD, Petrovitch H, et al. Association of olfactory dysfunction with incidental Lewy bodies. Mov Disord. 2006;21(12):2062–2067.
- Beach TG, Adler CH, Sue LI, et al. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol. 2008;115(4):445–451.
- Dickson DW, Fujishiro H, DelleDonne A, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol. 2008;115(4):437–444.
- DelleDonne A, Klos KJ, Fujishiro H, et al. Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol. 2008;65(8):1074–1080.
- Driver-Dunckley E, Adler CH, Hentz JG, et al. Olfactory dysfunction in incidental Lewy body disease and Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(11):1260–1262.
- Beach TG, Sue LI, Walker DG, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9(3):229–245.
- Beach TG, Adler CH, Sue LI, et al. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology. 2015;35(4):354–389.
- Beach TG, Adler CH, Lue L, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117(6):613–634.
- Walker DG, Lue LF, Adler CH, et al. Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Exp Neurol. 2013;240:190–204.
- McKinnon J, Evidente V, Driver-Dunckley E, et al. Olfaction in the elderly: a cross-sectional analysis comparing Parkinson’s disease with controls and other disorders. Int J Neurosci. 2010;120(1):36–39.
- Heinzel S, Berg D, Gasser T, et al. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2019;34(10):1464–1470.
- Friederich A, Flinspach A, Suenkel U, et al. Prodromal features of Parkinson’s disease: self-reported symptoms versus clinically assessed signs. Mov Disord. 2019;34(1):144–146.
- Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211.
- Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119(6):689–702.
- Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38(8):1237–1244.
- Shill HA, Hentz JG, Caviness JN, et al. Unawareness of hyposmia in elderly people with and without Parkinson’s disease. Mov Disord Clin Pract. 2016;3(1):43–47.


