 by David G. Daniel, MD; Alan Kott, MD; Jay Saoud, Phd; Remy Luthringer, PhD; Vadym Rud, MD, PhD; Andrii Skyrpnikov, MD, PhD; Rodica Stan, MD, PhD; Veselin Palazov, MD; Xingmei Wang, MS; and Michael Davidson, MD
by David G. Daniel, MD; Alan Kott, MD; Jay Saoud, Phd; Remy Luthringer, PhD; Vadym Rud, MD, PhD; Andrii Skyrpnikov, MD, PhD; Rodica Stan, MD, PhD; Veselin Palazov, MD; Xingmei Wang, MS; and Michael Davidson, MD
Dr. Daniel is with Signant Health in Mclean, Virginia. Dr. Kott is with Signant Health in Prague, the Czech Republic. Drs. Luthringer, Davidson, and Saoud are with Minerva Neurosciences in Waltham, Massachusetts. Drs. Rud and Skyrpnikov are with the Department of Psychiatry, Ukranian Medical Academy in Poltava, Ukraine. Dr. Stan is with the County Hospital in Piatra Neamt, Romania. Dr. Palazov is with the Clinic of Psychiatry Mental Health in Burgas, Bulgaria. Ms. Wang is with Signant Health in Wayne, Pennsylvania.
FUNDING: Funding was provided by Signant Health and Minerva Neurosciences.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Patients with schizophrenia who, prior to inclusion in placebo-controlled trials, experience the most severe and/or unstable symptoms might be more likely to manifest symptomatic worsening upon antipsychotic discontinuation.
Methods. This retrospective analysis included all randomized patients assigned to placebo (n=83) in a 12-week, double-blind, placebo-controlled outpatient trial of MIN-101 (roluperidone) for the treatment of negative symptoms in schizophrenia. The following risk factors were defined for exacerbation: instability between screening and baseline defined operationally as patients with the highest 10 percent of absolute change from the screening visit to baseline in the Positive and Negative Syndrome Scale (PANSS) total or one of the five PANSS Marder factors; screening or baseline severity in PANSS total or one of the five PANSS Marder factors; and gender and age. We used two operational criteria of relapse and the odds ratios of meeting the relapse criteria were calculated for each risk factor.
Results. The odds of meeting one of the operational thresholds for relapse after antipsychotic discontinuation were not statistically significantly increased in the subjects who were unstable on the PANSS total or on one of the five PANSS Marder factors before antipsychotic discontinuation. Further, the severity of PANSS total and Marder factor scores at screening and baseline were not statistically significantly associated with odds of relapse. Neither age nor gender had any effect on relapse rates.
Conclusion. Mild to moderate symptomatic variations in the severity of symptoms during screening and more severe symptomology at baseline as measured by the PANSS were not predictive of increased risk of subsequent relapse in schizophrenic patients.
KEYWORDS: Antipsychotic medication, placebo-controlled trial, Positive and Negative Syndrome Scale, schizophrenia
Innov Clin Neurosci. 2020;17(27–29)
The realization that negative symptoms are at least as burdensome as psychosis for individuals suffering from schizophrenia has led investigators in academia and the pharmaceutical industry to focus research efforts on better understanding these illness aspects. To reduce the confounding effects of psychosis and agitation, most but not all published trials targeting negative symptoms have involved patients who are symptomatically stable patients and used an add-on design in which the experimental compound or placebo was added to an antipsychotic. A potential disadvantage of the add-on design is the risk of confounding effects of antipsychotics, which might cause secondary negative symptoms. In contrast, a potential risk of the monotherapy design, i.e. experimental versus placebo, is the potential for symptomatic worsening and exacerbation. This is particularly relevant since most trials targeting negative symptoms are conducted in out-patient settings and tend to last 3 to 6 months.1,2 Regardless of the add-on or monotherapy designs, to reduce the risk of including patients who are unstable or who might undergo rapid symptomatic exacerbation into trials, an observation period is built into the trial design. It is hypothesized that patients whose symptoms vary during the 1- to 3-week observation period preceding randomization—beyond a predetermined threshold—are more likely to destabilize. By trial design, these patients are excluded from the trial. However, given that the hypothesis that more severely ill or unstable patients are more likely to undergo symptomatic exacerbation aligns with clinical common sense, the hypothesis is rarely tested and has never been evaluated in patients with predominately negative symptoms.
Methods
This retrospective analysis included all randomized patients assigned to placebo (n=83) in the double-blind, placebo-controlled outpatient trial of MIN-101 (roluperidone) for the treatment of negative symptoms in schizophrenia. A detailed description of the trial design and patient population can be found elsewhere.3 79 subjects had at least one post baseline visit. Subjects in the trial were required to be 18 to 60 years of age, inclusive; meet the diagnostic criteria for schizophrenia as defined in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition; be stable in terms of positive and negative symptoms of schizophrenia over the last three months; and have a Positive and Negative Syndrome Score (PANSS) negative subscore of at least 20 points. The screening period lasted up to 28 days. After at least two days of washout from all psychotropic medications, patients were randomly assigned to receive placebo or experimental medication for 12 weeks.
At baseline, the mean total PANSS score was 80.5 points and, for the five PANSS Marder factors,4 the mean uncontrolled hostility/excitement factor score was 6.8 points, the mean anxiety/depression factor score was 8.7 points, the mean positive symptom factor score was 19.4 points, the mean disorganized thought factor score was 20.5 points, and the mean negative symptom factor score was 25.1 points.
For the purposes of this retrospective analysis, subjects at risk for exacerbation were hypothesized to be those with the highest 10 percent of absolute change from the screening visit to baseline in the PANSS total or one of the five PANSS Marder factors. Additionally, we tested for the effect of screening or baseline severity in PANSS total or any of the five PANSS Marder factors as well as for the effect of age and gender on relapse rates. For analysis purposes, we operationally defined two criteria of relapse: (1) a 20-percent increase in PANSS total or one or more of the five PANSS Marder factors, a worsening of the Clinical Global Impression—Severity (CGI-S) score by at least three points, or a CGI—Improvement (CGI-I) score of six or seven points at two consecutive postbaseline visits excluding the first postbaseline visit; or (2) a stricter definition requiring a minimum of 30-percent increase in PANSS total or at least one of the five PANSS factors, a worsening of the CGI-S score by three points, or a CGI-I score of six or seven points at two consecutive postbaseline visits excluding the first postbaseline visit.
In the first step, logistic regression was performed to assess the effect of individual risk factors on relapse. In the subsequent step, we performed an age- and gender-corrected logistic regression on the individual risk factors. The analyses were carried out using SAS 9.4 (SAS Institute, Cary, NC, USA). Results are presented in odds ratios (ORs) for each risk factor described above. Given the post-hoc nature of the analysis and the relatively large number of tests performed, the alpha value of significance was corrected using Bonferroni correction, dividing the alpha value of 0.05 by the number of tests conducted within an analysis to determine the threshold of statistical significance.
Results
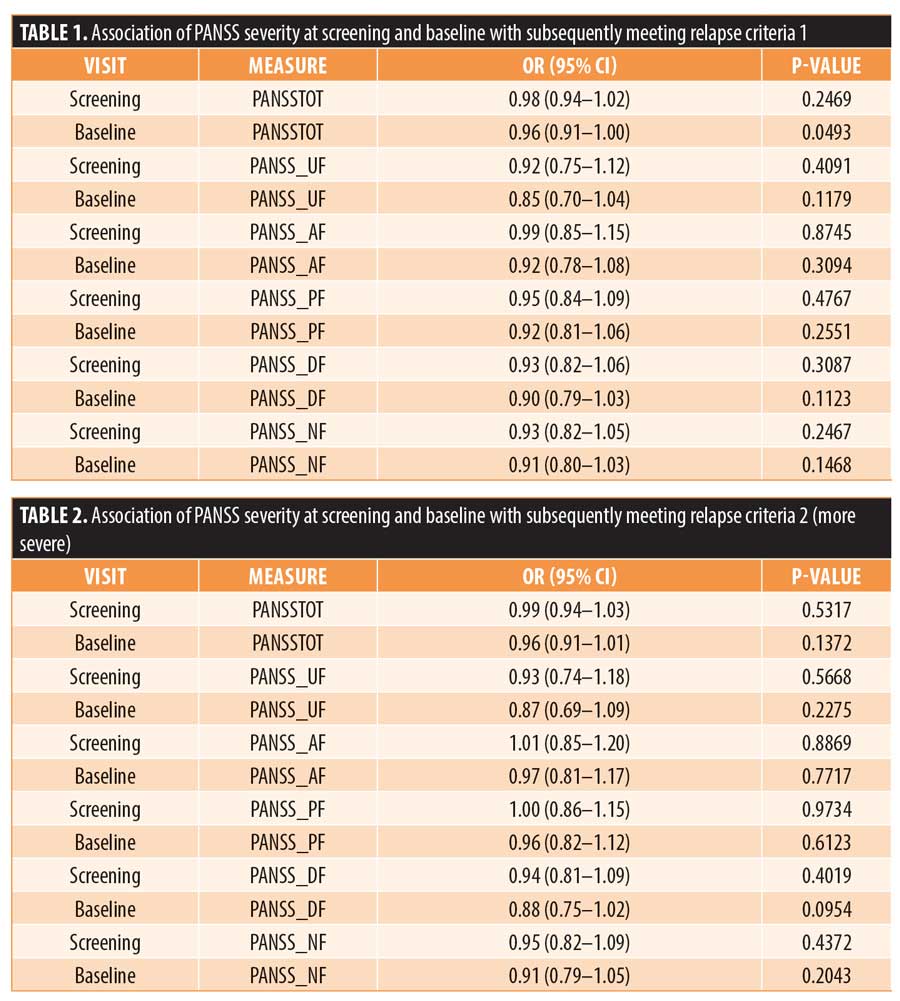
Depending on the definition of relapse, 26 or 17 subjects met the operational criteria for relapse based on worsening of their PANSS total, worsening of one of the five Marder factors, or worsening of the CGI scores. As shown in Figures 1 and 2, the odds of meeting one of the operational thresholds for relapse, regardless of how strict the definition was, were not statistically significantly increased among the subjects who were most unstable in the review of the PANSS total score or that of one of the five PANSS Marder factors. Relapse rates were also not affected by a subject’s age or gender.
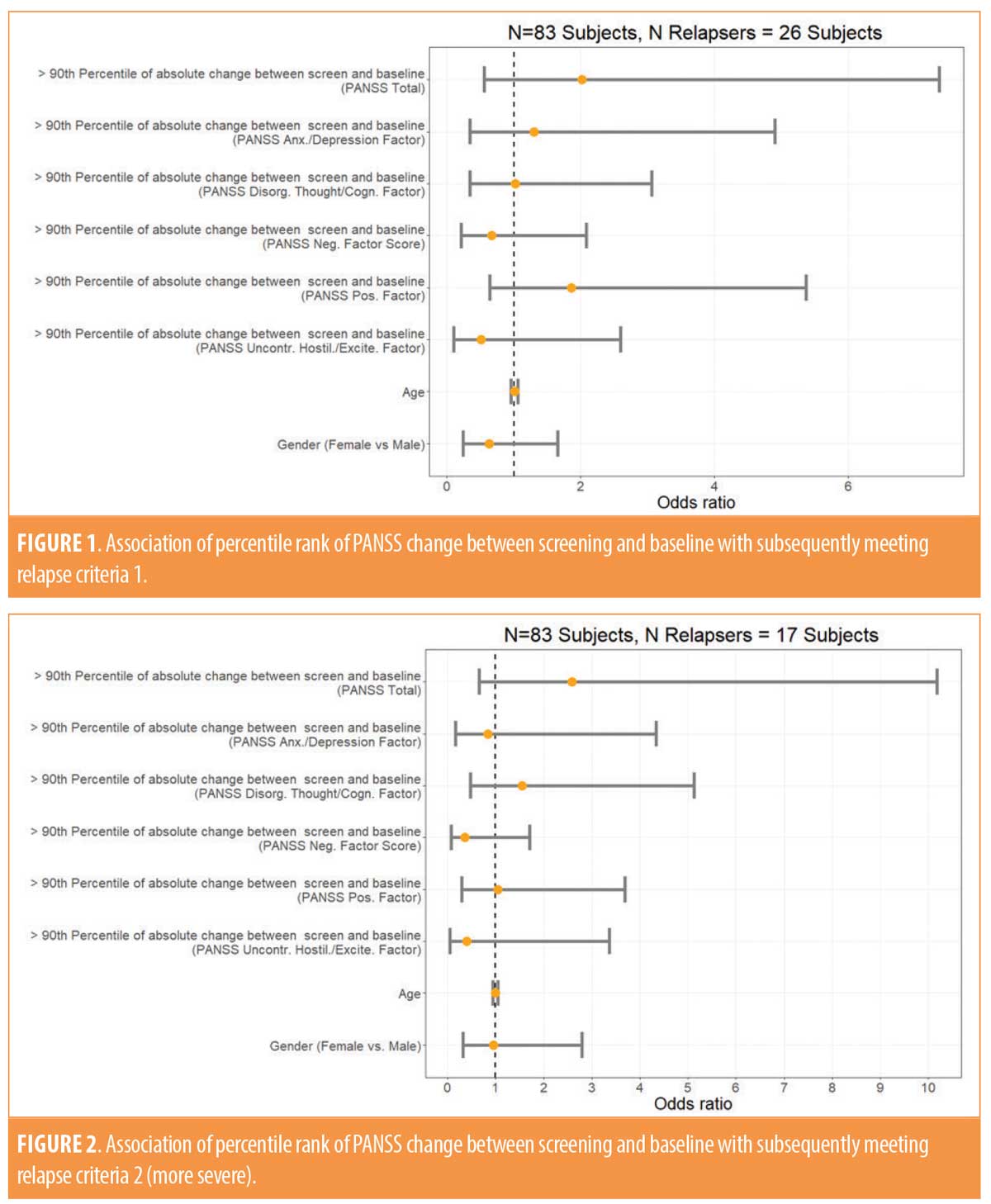
The severity of the PANSS total score at baseline was statistically significantly associated with meeting the milder relapse criteria prior to the application of the Bonferroni correction for multiple comparisons (OR: 0.95, 95% confidence interval: 0.91–1.00; p=0.0493). Following the application of the correction for multiple comparisons (i.e., dividing the alpha value of 0.05 by 12 given the number of tests performed), the p-value was found not to be significant. As shown in Tables 1 and 2, none of the other PANSS measures at screening or baseline were statistically significantly associated with either milder or more severe operationally defined relapse. No differences in the results were observed when we corrected for age and gender (data referenced in Appendix).
Discussion
In this post-hoc analysis of stable clinical trial subjects with moderate to severe negative symptoms who were washed out of their previous antipsychotic medications and assigned to a placebo for 12 weeks as outpatients, neither the instability of psychopathology during the screening period nor age or gender were associated with an increased risk of subsequently meeting the operational relapse criteria.
The severity of psychopathology at screening or baseline measured by the five Marder factors was also not statistically significantly predictive of increased relapse in this placebo-treated population. The severity of the total PANSS score at baseline was weakly but statistically significantly associated with meeting the milder relapse criteria (OR: 0.95, 95% confidence interval: 0.91–1.00; p=0.0493), but statistical significance did not survive the conduct of a Bonferroni correction for multiple comparisons. An association of milder psychopathology with modestly higher odds of relapse would be contrary to clinical experience and might reflect a chance finding or the phenomenon of regression to the mean. The results did not change when data were analyzed while correcting for age and gender.
Studies attempting to predict relapse for stable patients with predominately negative symptoms who are withdrawn from antipsychotics are of the upmost importance. These trials tend to be lengthy (typically 12–24 weeks) and are preferentially conducted in the outpatient community. However, mild to moderate symptomatic variations during screening and a greater severity of symptoms as measured by the PANSS were not predictive of increased risk of relapse in this predominantly negative symptom sample surveyed over 12 weeks of a double-blind placebo-controlled treatment. These findings are tentative due to the relatively modest sample size and relatively small number of relapses. Involving larger placebo-treated samples will be useful in making more definitive interpretations.
References
- Marder SR, Daniel DG, Alphs L, Awad AG, Keefe RS. Methodological issues in negative symptom trials. Schizophr Bull. 2011;37(2):250–254.
- Marder SR, Alphs L, Anghelescu IG, et al. “Issues and perspectives in designing clinical trials for negative symptoms in schizophrenia.” Schizophr Res. 2013;150(2-3):328–333.
- Davidson MJ, Saoud C, Staner N, et al. Efficacy and safety of MIN-101: a 12-week randomized, double-blind, placebo-controlled trial of a new drug in development for the treatment of negative symptoms in schizophrenia.” Am J Psychiatry. 2017;174(12):1195–1202.
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58(12):538–546.




