 by Tiffany Y. Lin, MD; Jasmine Hanna; and Waguih William IsHak, MD, FAPA
by Tiffany Y. Lin, MD; Jasmine Hanna; and Waguih William IsHak, MD, FAPA
Drs. Lin and Ishak are with the David Geffen School of Medicine at UCLA in Los Angeles, California. Dr. Ishak is also with the Department of Psychiatry & Behavioral Neurosciences at Cedars-Sinai Medical Center in Los Angeles, California. Ms. Hanna is a BA candidate at the University of California in Los Angeles, California.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. Psychiatric symptoms are frequently comorbid with Cushing’s syndrome (CS), a relatively rare condition that results from chronic hypercortisolism. Psychiatric manifestations might be present in the prodromal phase, during the course of the illness, and even after the resolution of CS. Our goals are to review the prevalence of psychiatric symptoms in CS; to determine the impact of psychiatric symptoms on morbidity, functioning, and quality of life; and to analyze the impact of treatment of CS on psychiatric symptoms.
Methods. A systematic search of the literature database was conducted according to predefined criteria. Two authors independently conducted a focused analysis of the full-text articles and reached a consensus on 17 articles to be included in this review.
Results. Overall, studies suggested that psychiatric symptoms—including, most prominently, depression—were present in a significant proportion of patients with CS. They reported lower health-related quality of life, which persisted even following the resolution of hypercortisolism. Though treatment and cure of CS significantly improved psychiatric symptoms, some patients did not achieve complete resolution of psychiatric symptoms and required continued psychiatric treatment.
Conclusion. The majority of the literature indicates that psychiatric manifestations are an important part of CS and overall lower health-related quality of life and psychiatric symptoms can persist even after the cure of CS. This emphasizes the significance of early diagnosis for psychiatric management and stresses the importance of monitoring the long-term effects of neurocognitive and psychiatric symptoms and its impact on the quality of life, even after hypercortisolism resolution.
KEYWORDS: Cushing’s syndrome, hypercortisolism, depression, anxiety, quality of life
Innov Clin Neurosci. 2020;17(30–35)
Cushing’s syndrome (CS) is a relatively rare but serious condition that results from chronic hypercortisolism.1 It causes significant morbidity and mortality due to cardiovascular, metabolic, and psychiatric sequelae.2 In addition to the physical features of CS, such as weight gain, central obesity, facial plethora, hirsutism, muscle wasting, and weakness,3 psychiatric manifestations are also a significant part of the condition. A significant proportion of patients with CS experience psychiatric symptoms—largely depression and anxiety disorders—and, although treatment of the underlying hypercortisolism might improve psychiatric symptoms, complete remission of these symptoms might not always be achieved.1
Because psychiatric symptoms might precede, occur during, or remain after the duration of illness,4,5 it is particularly important to recognize that psychiatric manifestations might be the presenting symptom of CS, to monitor psychiatric symptoms throughout disease course, and to continue to follow up on symptom severity and quality of life measures even after resolution of hypercortisolemia. The aims of this study were to review the prevalence of psychiatric symptoms in CS; to determine the impact of psychiatric symptoms on morbidity, functioning, and quality of life; and to analyze the impact of treatment of CS on psychiatric symptoms.
Methods
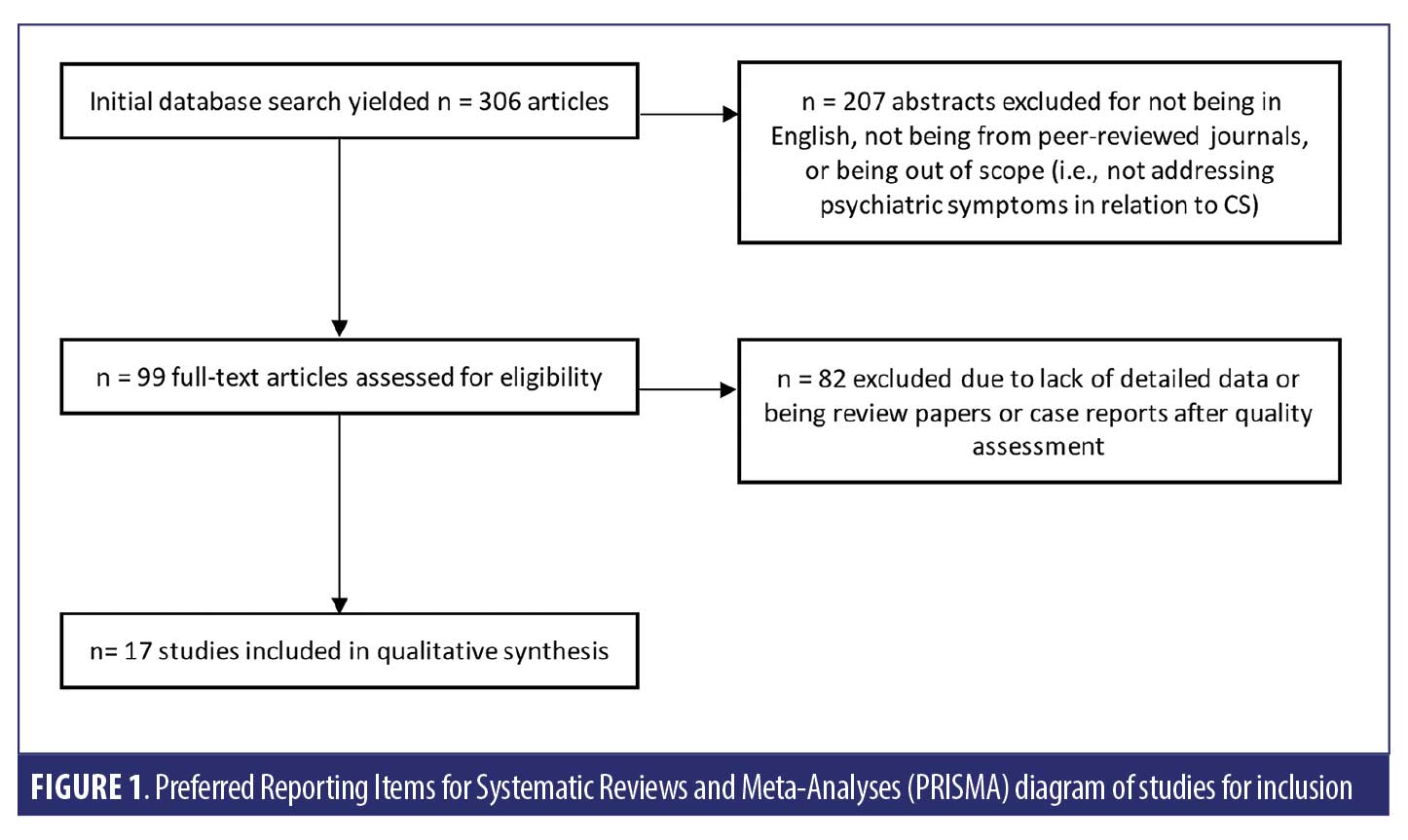
We adhered to recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement,6,7 including designing the analysis before performing the review. However, we did not perform a meta-analysis.
Study selection criteria and methodology. We searched the PubMed database for articles published until the time of search (March 2018). In PubMed, one search term string (“psych*” AND “Cushing*” AND “syndrome”) was used to capture studies of psychiatric symptoms in patients with CS. We included literature with no time limitation to encapsulate all the relevant studies about CS, a relatively rare condition. This initial search yielded 306 relevant articles. Two investigators independently reviewed the abstracts of these studies using the following criteria for inclusion: (1) articles written in English or with an available published English translation; (2) publication in a peer-reviewed journal; (3) studies of patients older than 18 years old; (4) studies that focused on psychiatric symptoms in CS. After eliminating duplicates and abstracts not suitable for inclusion, 99 articles met all these specific criteria. Both reviewers then independently conducted a focused review using the full text of these articles. The reviewers next reached a consensus on which studies to include in this review based on the article’s relevance to the stated goals of this review.
Data extraction. This selection process yielded 17 studies that met the full criteria described above. Two investigators reviewed each full-text article to extract information related to study design, population, and outcomes regarding clinical results. The data on relevant clinical outcomes included psychiatric symptom prevalence, symptom severity, quality of life, and daily functioning. See Figure 1 for an illustration of the search strategy. Research methodology and key findings were derived from the full text and tables of the selected studies.
Results
The results are depicted in Table 1.
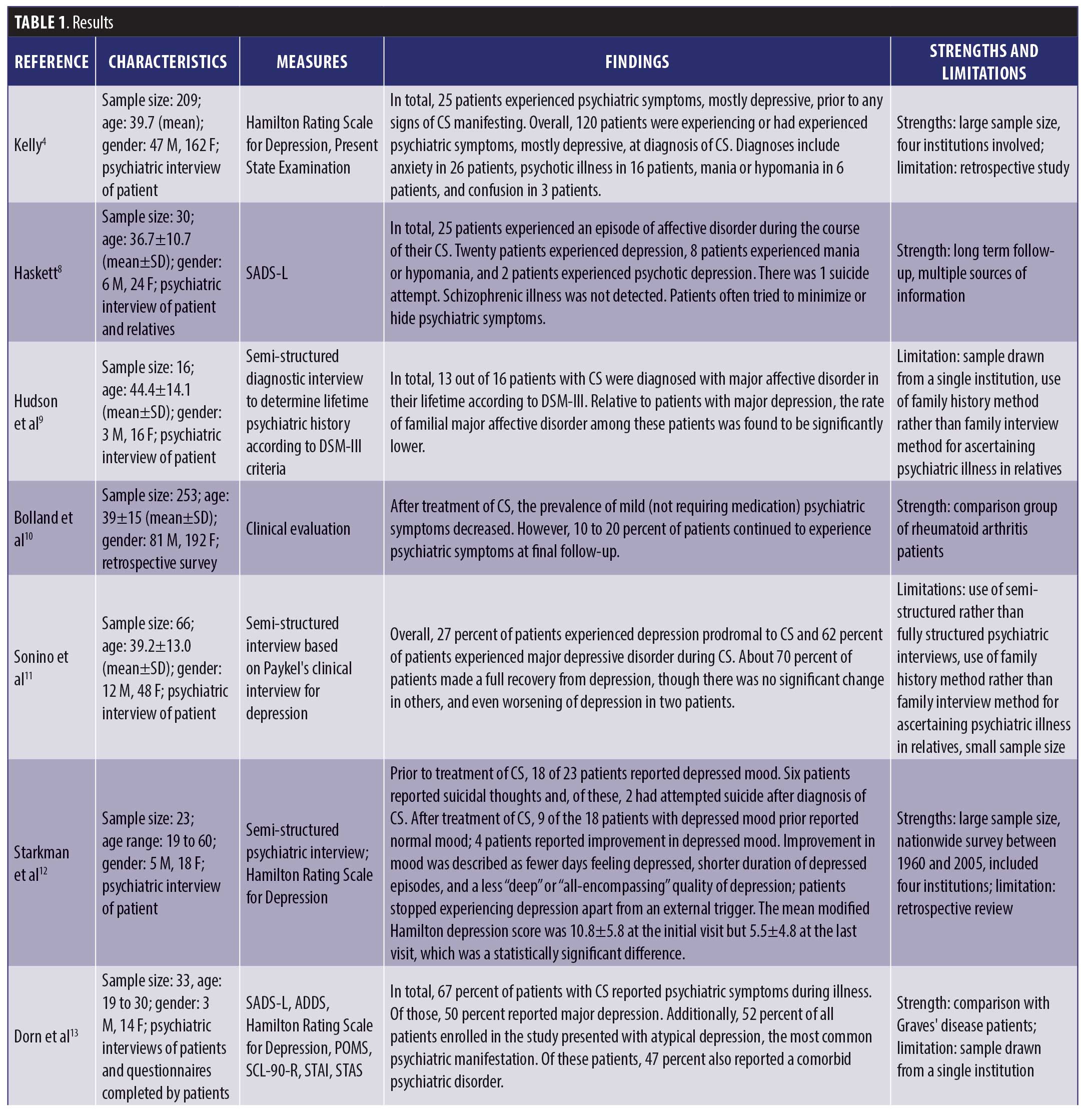
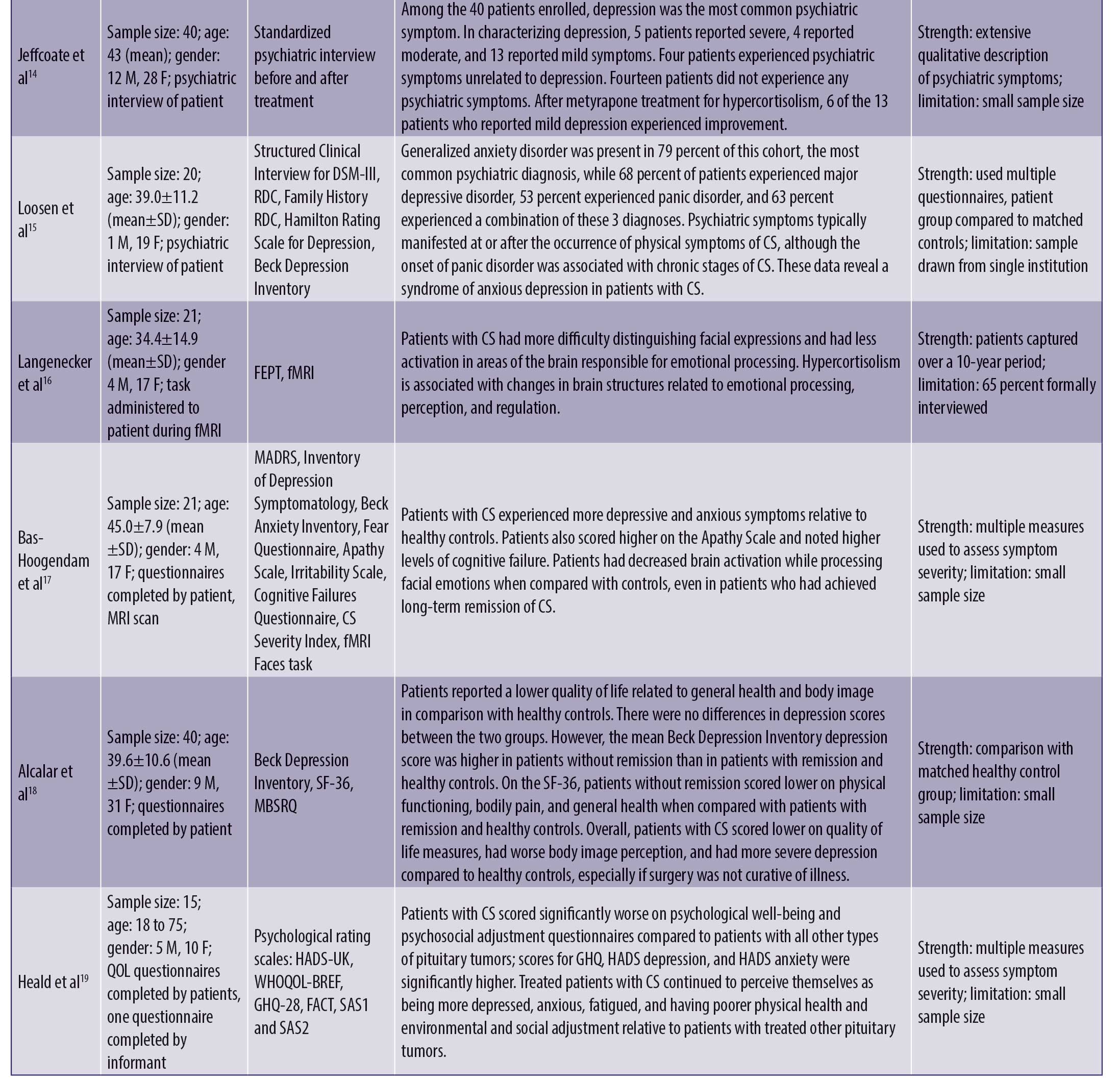
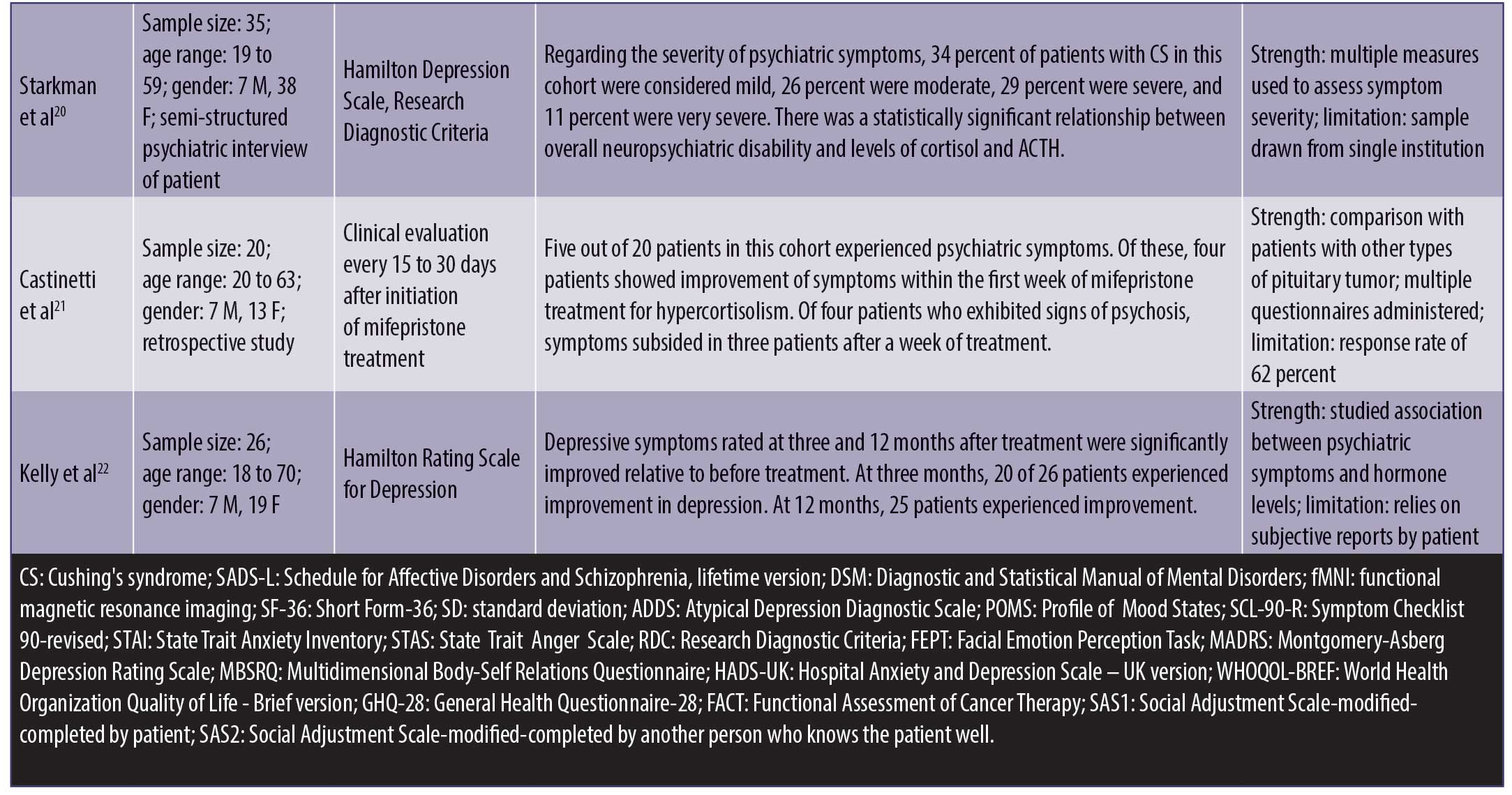
Psychiatric symptoms. Psychiatric symptoms were present in 20 to 83 percent of patients with CS.4,8–15 These psychiatric manifestations included depression (55–81%),4,8,9,11–15 anxiety (12%),4 mania or hypomania (3–27%),4,8 confusion (1%),4 psychosis (8%),4 and panic disorder (53%).15 These symptoms were present in the prodromal phase, during the course of the illness, and even persisted after resolution of CS.4 Research found that patients with CS made more errors in categorizing facial expressions and had less activation in emotion-processing centers of the brain due to cortisol dysregulation.16 This was true even in patients who had been cured of CS for many years.17
Quality of life. Studies showed that patients with CS had an overall lower health-related quality of life, with lower body image perception and higher levels of depression relative to health controls, particularly if the disease persisted despite surgery.18 In comparison with patients with other forms of pituitary tumors, patients with CS had higher scores for general health, depression, and anxiety questionnaires. They also continued to experience problems in multiple domains of life after cure and perceived themselves as more depressed, anxious, and fatigued as well as exhibiting poorer physical health and environmental and social adjustment.19 Regarding impact on functioning, one study found 34 percent of patients with mild, 26 percent with moderate, 29 percent with severe, and 11 percent with very severe psychiatric disability.20
Impact of treatment. Overall, studies found that, though the treatment and cure of CS significantly improved psychiatric symptoms, 10 to 28 percent of patients did not achieve complete resolution of psychiatric symptoms.10,12 For patients who used mifepristone to control hypercortisolemia during the course of illness and prior to surgical cure, 80 percent of patients experiencing psychiatric symptoms reported improvement within the first week of mifepristone use.21 For depressive symptoms specifically, several studies used the Hamilton rating scale to assess depressed mood, and 72 to 96 percent of affected patients reported statistically significant improvements in their score after the treatment of CS.12,22 Improvements of depressed mood were characterized by a decrease in the frequency of days in which patient felt depressed, each episode lasting for a shorter period of time, and a change in the quality of depressive mood.12 Full recovery occurs in about 75 percent of depressed patients after the cure of CS.11 For patients who used metyrapone to treat hypercortisolemia, depressive symptoms resolved in all markedly/severely depressed, in 75 percent of moderately depressed, and in 50 percent of mildly depressed patients.14
Discussion
The aims of these previous studies were to review the prevalence of psychiatric symptoms of CS; discuss various proper treatments for the patients affected by psychiatric symptoms due to hypercortisolism; emphasize the significance of early diagnosis for psychiatric management and necessary treatment administered in a timely manner for the resolution of hypercortisolism; and stress the importance of monitoring the long-term effects of neurocognitive symptoms, such as concentration and memory problems, in addition to psychiatric symptoms and its impact on the quality of life even after hypercortisolism resolution. In all of the studies reviewed on the psychiatric issues caused by CS, depression seemed to be the most prevalent and significant psychiatric disorder among patients with CS. As many as 90 percent of patients with CS suffer from depression due in part to exposure to excessive and extensive cortisol production from the adrenal glands or prolonged steroid drug use.23 Depression is related to the psychological impact of having an impairing and serious illness as well. Moreover, depression is associated with the effect of CS on self-esteem due to one’s self-degradation of body image because of the adverse physical symptoms of CS, such as weight gain around midsection, the development of a hump between the shoulders, facial plethora, hirsutism, muscle wasting, and weakness. CS leads to additional psychiatric disorders, such as anxiety (second most common), hypomania (third most common), psychosis, and mania.5 Clinicians should consider CS as a differential diagnosis when determining the diagnosis of a new-onset psychiatric illness or prolonged psychiatric symptoms. The reviewed studies show that the most important interventions are control of the cortisol level, lowering the high cortisol state, and the ultimate resolution of hypercortisolism to improve psychiatric and neurocognitive disorders, such as concentration and memory.5,23,24 However, it is important to note that, even after the resolution of hypercortisolism and the improvement of psychiatric and neurocognitive disorders, some patients still experience depression, anxiety, memory, and concentration problems due to residual effects of hypercortisolism on the brain.24 Psychiatric disorders—mainly depression as well as anxiety and mania—could become exacerbated after the resolution of hypercortisolism. The research studies show that psychiatric symptoms caused by CS have a detrimental impact on both physical and mental quality of life of patients with CS. Therefore, close and long-term monitoring of psychiatric and neurocognitive symptoms should be standard procedure in the management of CS both in the active phase of the illness and after remission. Although antiglucocorticoids and antidepressants have been suggested as effective choices for the treatment of depressive symptoms, psychotherapy is seen as an important treatment that is proven to be beneficial for depression and anxiety. Inhibitors of corticosteroid production, such as ketoconazole, metyrapone, and aminoglutethimide are generally successful in relieving depressive symptoms, along with other disabling symptoms, and may be used to control symptoms prior to surgical treatment.25,26 However, due to longstanding effects of hypercortisolism on the body and brain, treatment might still be required, even after the resolution of hypercortisolism. In these situations, antidepressants, such as tricyclic agents (e.g., amitriptyline, nortriptyline) and selective serotonin reuptake inhibitors (e.g., sertraline, fluoxetine, citalopram), in addition to cognitive behavioral therapies have been found to be particularly effective for depressive symptoms.25 In patients with severe anxiety, benzodiazepines, such as low-dose clonazepam, might be helpful.25
Literature review of studies that agree and disagree with the above findings. Previous authors have also found that psychiatric symptoms might precede CS by months or even years and, across a multitude of studies, the most prevalent psychiatric comorbidity of CS is depression,27–30 though mania and anxiety disorders have also been reported.29,30 Some study cohorts of patients with active CS report a majority of depressive symptoms, without cases of mania or anxiety.28 Additionally, there are several case reports describing CS with an acute psychotic presentation,31,32 though this is much rarer than other psychiatric manifestations. Psychiatric symptoms were found to be caused by anatomical and functional anomalies in the brain areas involved in processing of emotion and cognition, which are only partially restored after the biochemical remission of the disease.33 Broadly, several classes of drugs, including glucocorticoid receptor antagonists, steroidogenesis inhibitors, and pituitary tumor-targeted drugs used for medical treatment of CS, are also effective in treating the accompanying psychiatric symptoms.33 More specifically, in the treatment of depressive symptoms during the active phase of illness, the reduction of glucocorticoid synthesis or action with metyrapone, ketoconazole, or mifepristone, rather than treatment with antidepressant medications, is generally found to be successful.30,34 Many studies have found that, though the resolution of hypercortisolism improves the overall prevalence of the psychiatric and neurocognitive symptoms, patients might still experience impaired cognitive function and memory due to persisting brain volume loss.27,29,30,35–38 Prior studies reported a strong association between elevated cortisol levels and depression, hippocampal atrophy, and cognitive impairment,39 supporting the findings in our review. It seems to be the consensus that patients do not completely return to their premorbid level of functioning and quality of life and cognitive function continue to be impaired despite long-term cure.29,30,33,40 Furthermore, long-standing hypercortisolism might confer a degree of irreversibility to the pathological processes,29 and studies have shown that chronically elevated levels of glucocorticoids have detrimental effects on cognition,37,40–42 though there is also evidence suggesting that changes to the hippocampal region caused by hypercortisolism are at least partly reversible.43,44
Limitations. Few research studies have focused on the neurocognitive symptoms, such as concentration and memory impairments, affected due to the impact of a high-cortisol state on the hippocampus. Other common symptoms of CS, such as insomnia and fatigue, are also understudied. Head-to-head comparisons of treatment interventions of depressive symptoms in CS are also lacking, leading to difficulties faced by clinicians in guiding treatment for patients who do not readily respond to conventional interventions.
Conclusion
Early diagnosis of hypercortisolism aids in the process of normalizing excessive cortisol levels, thus preventing or lessening the severity of psychiatric symptoms in CS patients, especially if psychiatric symptoms are commonly the presenting features of CS. Going forward, a timely diagnosis and treatment initiation using antiglucocorticoids, antidepressants, and psychotherapy, monitoring the psychiatric symptoms of patients with CS during illness and postresolution of hypercortisolism are imperative to achieve the best results in resolving the psychiatric characteristics of CS and improving their quality of life.
References
- Santos A, Resmini E, Pascual JC, et al. Psychiatric symptoms in patients with Cushing’s syndrome: prevalence, diagnosis and management. Drugs. 2017;77(8):829–842.
- Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593–5602.
- Nieman LK. Cushing’s syndrome: update on signs, symptoms and biochemical screening. Eur J Endocrinol. 2015;173(4):M33–M38.
- Kelly WF. Psychiatric aspects of Cushing’s syndrome. QJM. 1996;89(7):543–551.
- Tang A, O’Sullivan AJ, Diamond T, et al. Psychiatric symptoms as a clinical presentation of Cushing’s syndrome. Ann Gen Psychiatry. 2013;12(1):23.
- Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013–1020.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Haskett RF. Diagnostic categorization of psychiatric disturbance in Cushing’s syndrome. Am J Psychiatry. 1985;142(8):911–916.
- Hudson JI, Hudson MS, Griffing GT, et al. Phenomenology and family history of affective disorder in Cushing’s disease. Am J Psychiatry. 1987;144(7):951–953.
- Bolland MJ, Holdaway IM, Berkeley JE, et al. Mortality and morbidity in Cushing’s syndrome in New Zealand. Clin Endocrinol (Oxf). 2011;75(4):436–442.
- Sonino N, Fava GA, Belluardo P, et al. Course of depression in Cushing’s syndrome: response to treatment and comparison with Graves’ disease. Horm Res. 1993;39(5–6):202–206.
- Starkman, MN, Schteingart, DE, Schork MA. Cushing’s syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19(3):177–188.
- Dorn LD, Burgess ES, Dubbert B, et al. Psychopathology in patients with endogenous Cushing’s syndrome: ‘atypical’ or melancholic features. Clin Endocrinol (Oxf). 1995;43(4):433–442.
- Jeffcoate WJ, Silverstone JT, Edwards CRW, Besser GM. Psychiatric manifestations of cushing’s syndrome: response to lowering of plasma cortisol. QJM. 1979;48(191):465–472.
- Loosen P, Chambliss B, DeBold C, et al. Psychiatric phenomenology in Cushing’s disease. Pharmacopsychiatry. 1992;25(4):192–198.
- Langenecker SA, Weisenbach SL, Giordani B, et al. Impact of chronic hypercortisolemia on affective processing. Neuropharmacology. 2012;62(1):217–225.
- Bas-Hoogendam JM, Andela CD, van der Werff SJ, et al. Altered neural processing of emotional faces in remitted Cushing’s disease. Psychoneuroendocrinology. 2015;59:134–146.
- Alcalar N, Ozkan S, Kadioglu P, et al. Evaluation of depression, quality of life and body image in patients with Cushing’s disease. Pituitary. 2013;16(3):333–340.
- Heald AH, Ghosh S, Bray S, et al. Long-term negative impact on quality of life in patients with successfully treated Cushing’s disease. Clin Endocrinol (Oxf). 2004;61(4):458–465.
- Starkman MN, Schteingart DE. Neuropsychiatric manifestations of patients with Cushing’s syndrome. Relationship to cortisol and adrenocorticotropic hormone levels. Arch Intern Med. 1981;141(2):215–219.
- Castinetti F, Fassnacht M, Johanssen S, et al. Merits and pitfalls of mifepristone in Cushing’s syndrome. Eur J Endocrinol. 2009;160(6):1003–1010.
- Kelly WF, Checkley SA, Bender DA, Mashiter K. Cushing’s syndrome and depression—a prospective study of 26 patients. Br J Psychiatry. 1983;142:16–19.
- Middleman D. Psychiatric issues of Cushing’s patients. https://csrf.net/coping-with-cushings/psychiatric-issues-of-cushings-patients/. Accessed March 10, 2020.
- Pivonello R, Simeoli C, De Martino MC, et al. Neuropsychiatric disorders in Cushing’s syndrome. Front Neurosci. 2015;9:129.
- Sonino N, Fava GA. Psychiatric disorders associated with Cushing’s syndrome. Epidemiology, pathophysiology and treatment. CNS Drugs. 2001;15(5):361–73.
- Obinata D, Yamaguchi K, Hirano D, et al. Preoperative management of Cushing’s syndrome with metyrapone for severe psychiatric disturbances. Int J Urol. 2008;15(4):361–362.
- Bratek A, Kozmin-Burzynska A, Gorniak E, Krysta K. Psychiatric disorders associated with Cushing’s syndrome. Psychiatr Danub. 2015;27:S339–S343.
- Kelly WF, Kelly MJ, Faragher BA. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol (Oxf). 1996;45(6):715–720.
- Sonino N, Fallo F, Fava GA. Psychosomatic aspects of Cushing’s syndrome. Rev Endocr Metab Disord. 2010;11(2):95–104.
- Pereira AM, Tiemensma J, Romijn JA. Neuropsychiatric disorders in Cushing’s syndrome. Neuroendocrinology. 2010;92 Suppl 1:65–70.
- Hirsch D, Orr G, Kantarovich V, et al. Cushing’s syndrome presenting as a schizophrenia-like psychotic state. Isr J Psychiatry Relat Sci. 2000;37(1):46–50.
- Wu Y, Chen J, Ma Y, Chen Z. Case report of Cushing’s syndrome with an acute psychotic presentation. Shanghai Arch Psychiatry. 2016;28(3):169–172.
- Bernini G, Tricò D. Cushing’s Syndrome and steroid dementia. Recent Pat Endocr Metab Immune Drug Discov. 2016;10(1):50–55.
- Wolkowitz OM, Reus VI, Manfredi F, et al. Antiglucocorticoid strategies in hypercortisolemic states. Psychopharmacol Bull. 1992;28(3):247–251.
- Burkhardt T, Ludecke D, Spies L, et al. Hippocampal and cerebellar atrophy in patients with Cushing’s disease. Neurosurg Focus. 2015;39(5):E5.
- Pivonello R, De Martino MC, De Leo M, et al. Cushing’s syndrome: aftermath of the cure. Arq Bras Endocrinol Metabol. 2007;51(8):1381–1391.
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol Psychiatry. 1992;32(9):756–765.
- Chen YF, Li YF, Chen X, Sun QF. Neuropsychiatric disorders and cognitive dysfunction in patients with Cushing’s disease. Chin Med J (Engl). 2013;126(16):3156–3160.
- Brown E, Varghese F, McEwen B. Association of depression with medical illness: does cortisol play a role? Biol Psychiatry. 2004;55(1):1–9.
- Resmini E, Santos A, Webb SM. Cortisol excess and the brain. Front Horm Res. 2016;46:74–86.
- Starkman MN, Giordani B, Berent S, et al. Elevated cortisol levels in Cushing’s disease are associated with cognitive decrements. Psychosom Med. 2001;63(6):985–993.
- Tiemensma J, Kokshoorn NE, Biermasz NR, et al. Subtle cognitive impairments in patients with long-term cure of Cushing’s disease. J Clin Endocrinol Metab. 2010;95(6):2699–2714.
- Starkman MN, Giordani B, Gebarski SS, et al. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s disease. Biol Psychiatry. 1999;46(12):1595–1602.
- Hook JN, Giordani B, Schteingart DE, et al. Patterns of cognitive change over time and relationship to age following successful treatment of Cushing’s disease. J Int Neuropsychol Soc. 2007;13(1):21–29.





