 by Kimberly B. Mulcahy, PharmD; Eileen Trigoboff, RN, DNS; Lewis Opler, MD, PhD; and Tammie Lee Demler, BS, PharmD, MBA
by Kimberly B. Mulcahy, PharmD; Eileen Trigoboff, RN, DNS; Lewis Opler, MD, PhD; and Tammie Lee Demler, BS, PharmD, MBA
Drs. Trigoboff, Demler, and Mulcahy are with Buffalo Psychiatric Center, New York State Office of Mental Health, and State University of New York, University at Buffalo School of Pharmacy and Pharmaceutical Sciences, Buffalo, New York; Drs. Trigoboff and Demler are with State University of New York, University at Buffalo School of Medicine, Department of Psychiatry, Buffalo, New York; and Dr. Opler is with the Predoctoral Program in Clinical Psychology, Long Island University, Brooklyn, New York.
Innov Clin Neurosci. 2016;13(5–6):21–27.
Funding: No funding was received for the preparation of this manuscript.
Financial Disclosures: The authors have no financial disclosures relevant to the content of this article.
Key words: Vitamin D, deficiency, psychiatric inpatients, schizophrenia, depression, multiple sclerosis, mood disorders, prescribing practices
Abstract: Vitamin D supplementation has become an increasingly popular prescribing practice, despite our limited knowledge of both the definition and degree of deficiency as well as the expected benefits or risks of exogenous administration. Many of the hypothesized benefits of vitamin D supplementation include a variety of improvements in mental health; however, these claims are not consistently or robustly supported by current research. In this paper, we provide a brief overview of what is currently known about vitamin D deficiency and about outcomes of supplementation as well as a summary of the data relative to prescribing practices for inpatients in an urban psychiatric hospital.
Background
Recent studies have shown a high prevalence of vitamin D deficiency in patients with psychiatric disorders, including schizophrenia, seasonal affective disorder, depression, and cognitive impairment.[1],[2] Many studies state the importance of screening for vitamin D deficiency in patients with major psychiatric illnesses due to the many effects of vitamin D on brain development, synaptic plasticity, neuronal development, and the protective factors against oxidative stress.[1–7] While a vitamin D guideline exists for management of vitamin D deficiency and bone health, there are no guidelines currently available for the use of vitamin D as an adjunctive treatment for other conditions.[2],[8] This study and literature review will examine prescribing practices, prescriber rationale for use, and the impact of vitamin D levels on psychiatric disorders.
Literature Review/ Introduction
Vitamin D deficiency is a worldwide problem affecting as many as one billion people, and it is estimated that 20 to 100 percent of American, Canadian, and European elderly men and women are deficient.[8],[9] According to the Endocrine Society’s Clinical Practice Guideline on the Prevention of Vitamin D Deficiency in 2011, a serum vitamin D (25[OH]D) level of 21 to 29ng/mL (52.5–72.5nmol/L) is insufficient, and a level less than 20ng/mL(50nmol/L) is considered deficient.[8] Sufficient vitamin D status is important for a variety of health concerns, including bone and muscle malfunctions, cardiovascular disease, some cancers, and autoimmune diseases.[1],[10],[11] Vitamin D is obtained through diet or exposure to the sun. However, only a few foods (e.g., salmon, mackerel, cod liver oil) contain vitamin D naturally, and geographic latitude, altitude, and season can affect the wavelength of sunlight and reduce the efficacy of the stimulation of our intrinsic production of vitamin D.12 Some individuals are at increased risk of vitamin D deficiency, including the elderly, those with obesity, individuals with dark skin pigmentation, and those with limited sun exposure. Due to the challenges and risks of prolonged sunlight exposure and inadequate dietary intake, supplements are often utilized to achieve sufficient vitamin D levels.[13]
Vitamin D is available as vitamin D2 (ergocalciferol), from plant and enriched dietary sources, and vitamin D3 (cholecalciferol), from fish, meat, and sunlight.[12],[14],[15] Our body metabolizes vitamin D in multiple steps in order for it to be distributed and utilized throughout the body. Initially it undergoes 25-hydroxylation in the liver by enzymes CYP2R1, 3A4, and 27A1 to 25-hydroxycholecalciferol (25(OH)D), the main form of vitamin D in our circulation.12 Recent reports found the CYP27A1 enzyme in the kidneys, intestines, bone, skin, lung, spleen, and central nervous system (CNS) in rats, suggesting metabolism and availability of vitamin D’s major metabolite to be greater than possibly thought in humans.[14] Next 25D is converted to active steroid hormone 1,25-dihydroxycholecalciferol (1,25(OH)2D) by CYP27B1 in the kidney.[12],[15] Serum concentration of 25(OH)D is used to obtain vitamin D status due to the body’s narrow regulation of 1,25(OH2)D with only picomolar quantities available in the circulation. The chronic use of medications that undergo CYP3A4 metabolism, including antiepileptics, glucocorticoids, antiretrovirals, and antifungals, and medications that interfere with fat absorption, such as bile acid sequestrants and lipase inhibitors, can interfere with vitamin D absorption, activation, and production.[8],[14],[16],[17]
Recent discovery of vitamin D receptors (VDR) in the brain have led to further explanation of the impact and contribution of vitamin D in psychiatric and neurologic development, conditions, and behavior. VDRs have been found in the hippocampus, cerebellum, and substantia nigra, which are the areas of the brain responsible for depression, schizophrenia, and other mood disorders.[5],[6],[7],[18–20] Furthermore, vitamin D activity it is considered to be involved in the modulation of the hypothalamic-pituitary-adrenal (HPA) axis which regulates the production of the neurotransmitters epinephrine, norepinephrine, and dopamine in the adrenal cortex, and also protects against the depletion of dopamine and serotonin.[5–7],[18] Based on the location in the key brain areas and effects on neurotransmitters that regulate behavior, vitamin D deficiency has been associated with irritability, anxiety, depression, psychoses, and deficits in mental development. Vitamin D is also a neurosteroid hormone, which may include functions such as the regulation of neurotransmission, neuroprotection and neuroimmunomodulation.[4–7],[18],[20],[21]
The neuroprotective effects of vitamin D are a result of multiple mechanisms, including the reduction of calcium toxicity and nitric oxide synthesis, modulation of glutathione metabolism and cytokine release, protection of neurons from induced cell death, and direct antioxidant properties. The neuroimmuno-modulating properties of vitamin D are likely due to altered cytokine, macrophage, dendritic and T cell expression, functions, and sensitivity to apoptic signals.[7] Despite the known information relative to its biologic implications, the relationship between vitamin D deficiency and major mental disorders is not well understood.[9]
Depression is prevalent worldwide and causes significant morbidity and mortality.[22] Many studies show current pharmacologic treatments have modest efficacy compared to placebo, resulting in healthcare providers and patients searching for other options in therapy.[22] Vitamin D has recently been explored as a potential treatment option due to studies revealing a correlation between low vitamin D levels and depressive symptoms.
A recent meta-analysis on observational studies concluded the risk of depression decreases by 10 percent for every 10ng/mL increase in 25(OH)D serum levels.[4] A study by Polak et al in 2014[5] reinforced these findings by noting the Center for Epidemiologic Studies Depression Scale (CES-D) scores are negatively associated with vitamin D levels, even when controlling for patient demographics and time spent outdoors. In contrast, another recent meta-analysis by Li et al in 2013[22] that evaluated randomized, controlled trials investigating the efficacy of vitamin D therapy in depression did not find an effect of supplementation on depression symptoms. Furthermore, an observational study4 conducted on geriatric patients, a population already at increased risk for vitamin D deficiency and depression, found no independent correlation between vitamin D deficiency and depression over a 4-year period. Due to the amount of heterogeneity between studies as well as lack of controls for outside variables, it is difficult to ascertain if there is in fact an independent correlation between depression and vitamin D deficiency or if the impact of vitamin D as a therapeutic intervention for depression is meaningful.[4],[22]
The few studies that explored vitamin D on human physiological functioning found an inverse linear association between 25(OH)D serum levels and ventricle size. It has also been found that vitamin D deficient neonatal rats had altered size and shape of the brain, altered growth factor expression and cell proliferation, enlarged ventricles, and cortical thinning, which are pathophysiologic traits associated with schizophrenia.[17],[18],[22],[23]
If vitamin D is in fact associated with schizophrenia, it is unknown what role it plays and how much of an implication it could have on patient recovery. Graham et al[3] compared vitamin D levels with symptom severity and cognition in patients with schizophrenia versus healthy controls. Utilizing the Positive and Negative Syndrome Scale (PANSS), more severe negative symptoms were associated with a low vitamin D level (p=0.03), and patients with insufficient vitamin D levels had significantly higher scores on the total PANSS in the subjects with schizophrenia. More severe cognitive defects were associated with lower vitamin D levels in patients with schizophrenia (p=0.003) but there was no association with the healthy controls.[3]
Serum 25(OH)D levels in patients with schizophrenia were compared to patients with depression and healthy control subjects in a study conducted by Itzhaky et al in 2012.[20] Patients with schizophrenia had significantly lower levels compared to patients with depression (p<0.05), but there was no difference between patients with depression compared to healthy controls.20 A similar study was conducted also comparing patients with schizophrenia, depression, and healthy controls.23 However, in this study healthy controls had significantly higher vitamin D levels than patients with schizophrenia (p=0.001) and also patients with depression (p<0.001), while the researchers found there was no difference between either the depression or schizophrenia group (p=0.563).[23]
Schizophrenia is a multifactorial disease and despite the possible correlation of vitamin D deficits in patients, further studies are required to determine if there is an increased risk of schizophrenia caused by vitamin D deficiency or if vitamin D supplementation can assist in symptom management.[7],[23]
A limitation within studies is the inability to control for additional outside factors that could also influence vitamin D levels. For instance, patients who are routinely hospitalized or have social withdrawal due to psychiatric illness may not be receiving adequate amounts of sunlight or not eating a proper diet and therefore not endogenously creating vitamin D, resulting in a misrepresentation of the claim of insufficient vitamin D levels with psychiatric disorders. Norelli et al in 201010 conducted a study that compared vitamin D levels in a psychiatric hospital of acute care patients (<6 months of stay) and long-stay patients (?6 months of stay) with a diagnosis of schizophrenia or schizoaffective disorders to an outpatient control group of healthy volunteers without mental illness who also worked at the facility. There was no statistically significant difference in vitamin D level in any group, even when controlled for age, sex, race or ethnicity, or body mass index (BMI) (n=60, p=0.316).[10] Abdullah et al11 supplied vitamin D supplementation to 290 long-term inpatients at a psychiatric facility, and despite raising serum levels to greater than 32ng/mL (defined as sufficient according to study protocol), there were no improvements in the Brief Psychiatric Rating Scale (BPRS) scores from baseline.
Patients with obesity or those with a high BMI are at increased risk of vitamin D deficiency because vitamin D is a fat soluble vitamin and there is a less discernable and available amount in circulation because it distributes to the body’s fat stores.[8] Many of the atypical antipsychotics and other mood stabilizing medications can cause weight gain and fat redistribution; this could confound results if deficiency is a primary disorder or due to the psychiatric illness, or rather an effect of treatment.[8],[24] Mood stabilizers and antiepileptic medications are also associated with vitamin D deficiency due to drug interactions, therefore making a possible false correlation between vitamin D levels and psychiatric diagnosis.[14],[24] These medications induce CYP3A4 and increased the activity of CYP34A, which increases the clearance of vitamin D metabolites and reduce vitamin D levels especially in patients with insufficient vitamin D exposure via diet, supplement, or ultraviolet (UV) light.13 It has also been noted that vitamin D can induce CYP3A4 and increase the expression of CYP3A4, CYP2B6, and CYP2C9 in hepatocytes by activating promoter genes, causing increased metabolism of many medications.[12] Due to the inconclusive evidence from recent studies, current guidelines only recommend the use of vitamin D supplementation to treat vitamin D deficiency.[8]
Polypharmacy in patients frequently results in decreased medication adherence, and the reduced cognitive organization and memory skills in patients with psychiatric diagnoses can further complicate adherence issues. Patients with a psychiatric diagnosis often have complex medication regimens, and in most diagnoses, medication adherence is required for remission. It is important to consider how a patient’s pill burden can reduce medication adherence as well as the importance of utilizing efficacious medications in the patient’s treatment.
Objectives
There were three hypotheses tested in this study. The primary hypothesis was to determine whether vitamin D was used in psychiatric inpatients when there was a deficiency confirmed with a serum 25(OH)D level. The second hypothesis was to determine if prescribers were using vitamin D supplementation as a prophylactic measure. Lastly, prescribing practices were evaluated to observe possible trends with vitamin D prescribing within a psychiatric hospital. Currently, there are no guidelines to support the use of vitamin D for treatment or management of psychiatric symptoms; this study investigated how prescribers utilized vitamin D within an inpatient psychiatric population.
Methods
The study was approved by the institutional review board of record for the facility. For the first hypothesis, we conducted a retrospective chart review of adult inpatients receiving vitamin D2 (ergocalciferol) in 2012, a random sample of adult in patients receiving ergocalciferol 10 years prior in 2002, and a random sample of patients who did not receive ergocalciferol in 2012 and 2002. Patient charts were evaluated for the receipt of ergocalciferol, presence of serum 25(OH)D levels and laboratory results, prescription order for supplementation and indication, duration of therapy, and discontinuation information if applicable. If patients had a 25(OH)D level in their chart, they were further categorized as deficient (2002 standards: <8.9ng/mL; 2012 standards: <32ng/mL) or sufficient (2002 standards: ?8.9ng/mL; 2012 standards: ?32ng/mL). A t-test was completed using the Statistical Package for Social Sciences version 22 (SPSS v22) to compare the average 25(OH)D levels in 2002 and 2012 based on receipt of supplementation. The level for statistical significance was selected at p<0.05. This level was selected to be more rigorous about significant outcomes relevant to the clinical outcomes and minimizing the risk of making a Type 1 error.
Hypotheses 2 and 3 were tested by an 11-question, voluntary, anonymous prescriber survey completed by inpatient physicians, psychiatrists, and nurse practitioners. Questions included determining preferred vitamin D formulation, indication for use, dose strength, and laboratory monitoring practices.
To be included in this study for the first hypothesis, patients had to be inpatients at the state psychiatric center at any time between January 1, 2002, and December 31, 2002, or January 1, 2012, and December 31, 2012, who were over the age of 18 years and did not have a Criminal Procedure Law (CPL) designation.
Healthcare providers who prescribed at Buffalo Psychiatric Center between January 1, 2002, and December 31, 2012, were offered the voluntary anonymous survey for Hypotheses 2 and 3. Healthcare providers who did not prescribe at the facility were excluded from completing the survey.
Hypothesis 1. Inclusion criteria.
• Male and female adults aged ?18 years
• Inpatient at the state psychiatric center at any time between January 1, 2002 and December 31, 2002, or January 1, 2012 and December 31, 2012 Exclusion criteria.
• Individuals with a Criminal Procedure Law (CPL) designation
Hypotheses 2 and 3. Inclusion criteria.
• Prescribed at Buffalo Psychiatric Center between January 1, 2002 and December 31, 2012
Exclusion criteria.
• Did not have prescribing privileges at Buffalo Psychiatric Center
Results
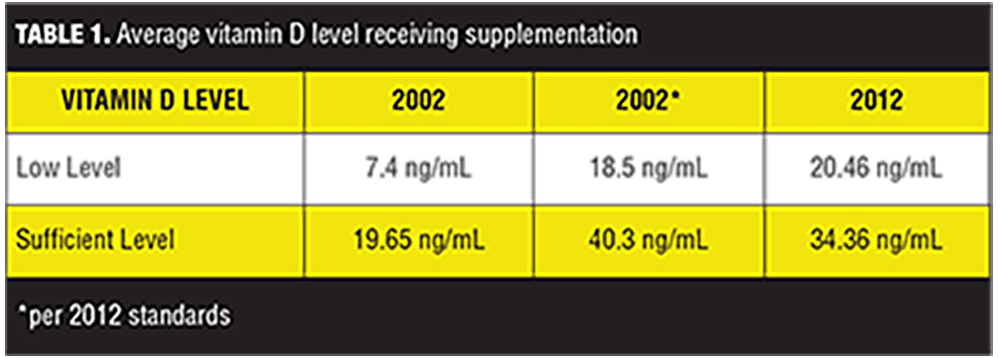
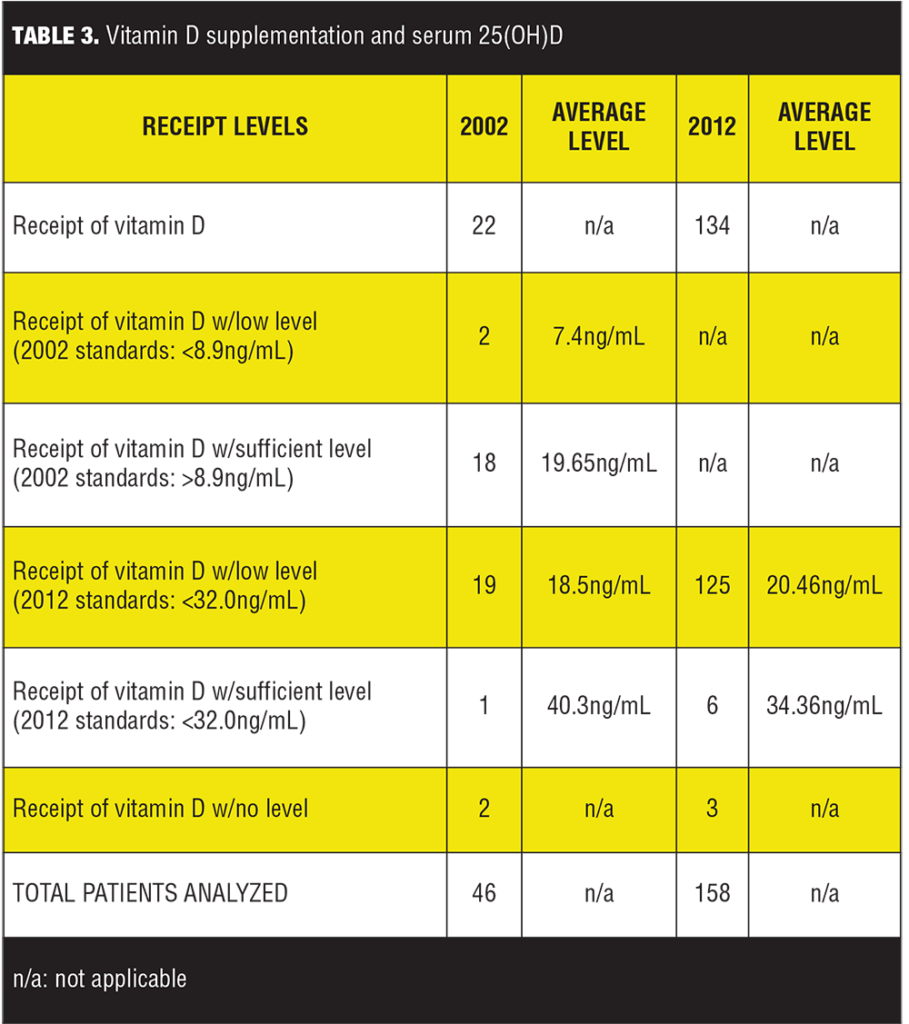
Charts of 204 patients were reviewed. A one-sample t-test was conducted to compare patients with both a deficient 25(OH)D level and who received supplementation with vitamin D in the years 2002 and 2012. In 2002, the average deficient level of patients receiving vitamin D was 7.4ng/mL; in 2012, the average deficient level of patients receiving vitamin D was 20.46ng/mL (Table 1). Despite different values, it was found that the difference between the average deficient levels of 25(OH)D in those given vitamin D supplementation in 2002 and the average low levels of those given vitamin D in 2012 were not statistically significantly different (p=0.128).
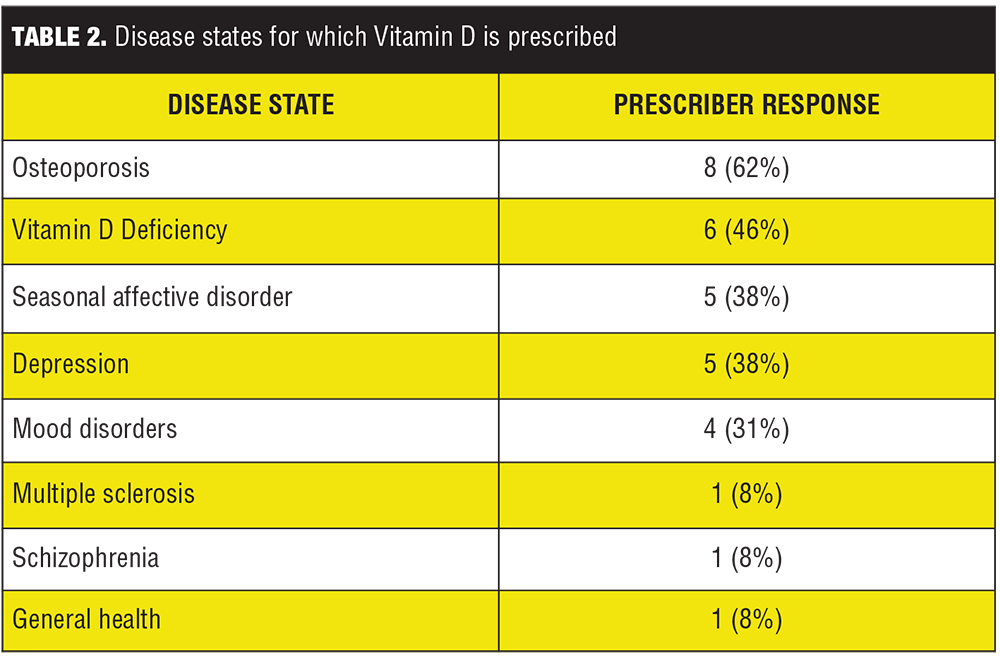
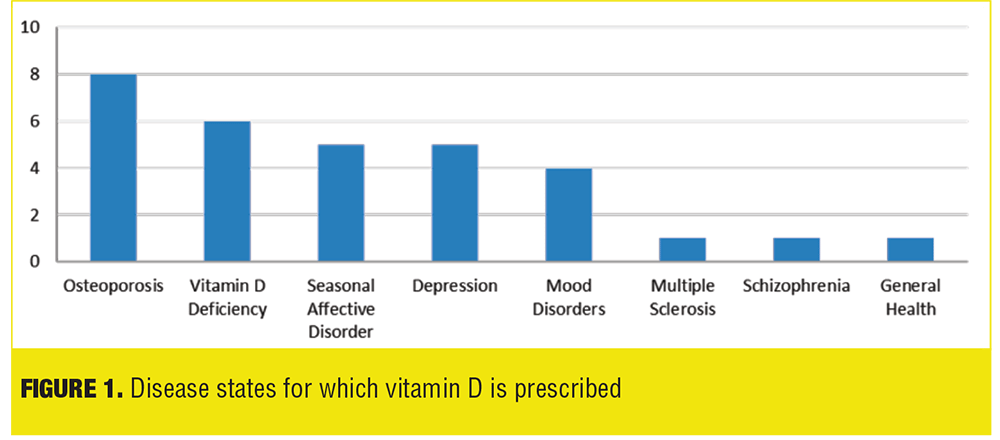
Data from the Prescriber Survey revealed a trend in prescribing practices in regard to vitamin D supplementation for psychiatric inpatients. The trended results show osteoporosis is the most common disease state (9 out of 13 prescribers, 62%); followed by vitamin D deficiency (6 out of 13 prescribers, 46%); depression and seasonal affective disorder (5 out of 13 prescribers, 38% each); mood disorders (4 out of 13 prescribers, 31%); and multiple sclerosis, seizures, and general health (1 out of 13 prescribers, 8% each) (Table 2, Figure 1). Only one prescriber out of 13 (8%) considered vitamin D supplementation a prophylactic measure.
Further findings included the amount of providers who obtained a baseline vitamin D level when admitting patients (7 out of 13 prescribers, 53%), the preferred formulation (Vitamin D2 8%, Vitamin D3 62%, and no preference 30%), frequency of serum monitoring (16% never, 0% every 1–2 months, 15% every 3-5 months, 39% every 6 months, 15% yearly, 15% other), and if guidelines were referenced (23% stated Yes). Prescriber’s definition of vitamin D deficiency was also assessed: one prescriber (8%) stated 19ng/mL or less as the cut off, six prescribers (46%) stated 24 ng/mL or less, five prescribers (38%) stated 29ng/mL or less, and one prescriber (8%) stated 34 ng/mL or less (Figure 3).
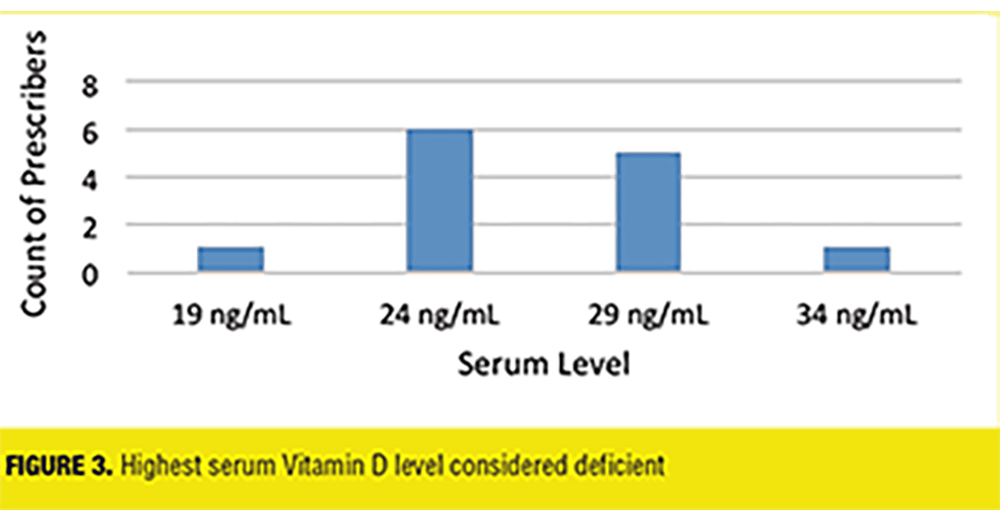
Discussion
Our results show no statistically significant difference between the average deficient 25(OH)D levels in patients supplemented with vitamin D in 2002 compared to 2012. An important factor to consider with this result is in 2002 the definition of vitamin D deficiency was less than 8.9 ng/mL, whereas in 2012 it was defined as less than 32.0ng/mL. The Institute of Medicine Food and Nutrition Board defined the lowest level of normal to be 8ng/mL in 1997 and in 2011 the Endocrine Society defined vitamin D sufficiency at levels above 30ng/mL, explaining the different laboratory deficiency definitions between the years 2002 and 2012. The lower vitamin D deficiency level in 2002 resulted in a higher percent of patients considered deficient receiving vitamin D supplementation despite having a sufficient level compared to 2012 (Table 3, Figure 2). If vitamin D standards were consistent between the years 2002 and 2012 utilizing the current 2011 Endocrine Society guidelines of less than 32.0ng/mL as deficient instead of less than 8.9 ng/mL, 19 patients (as opposed to 2 patients) would have deficient [25](OH)D level in 2002, and the average deficient level would be 18.5ng/mL (11.1ng/mL difference) (Table 1, Figure 1). Also, the number of patients analyzed differed significantly between 2002 and 2012 (46 and 158, respectively) due to the increased rate of vitamin D prescribing in 2012.
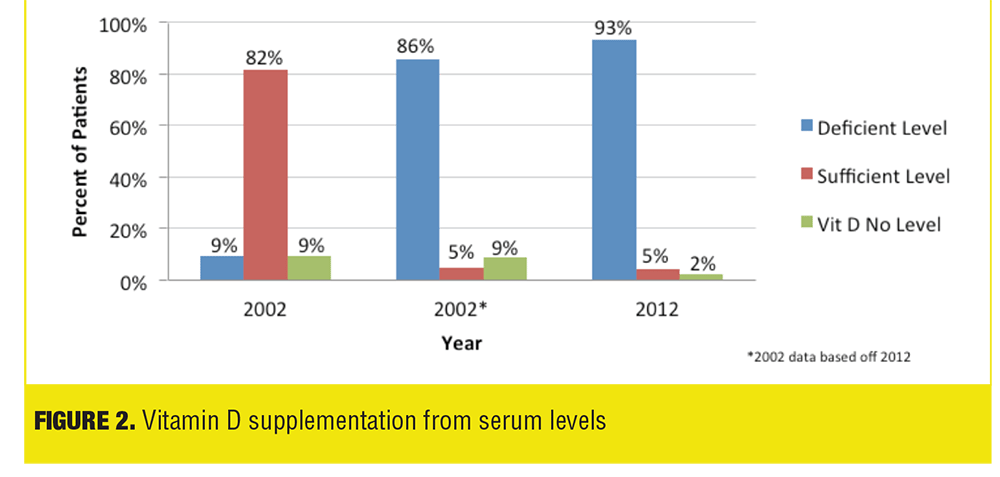
Majority of prescribers did not consider vitamin D supplementation as a prophylactic measure in the physician survey. This prescribing practice is confirmed not only by the survey but also the percentage of patients receiving vitamin D confirmed to have deficient serum 25(OH)D levels. Current guidelines from the Endocrine Society state vitamin D should be prescribed and monitored only in patients who are at risk for deficiency, indicating our prescribers are following best practice initiatives.[4]
The most common disease states for vitamin D supplementation were osteoporosis and vitamin D deficiency, which are the only indications for vitamin D in the guideline. However, a significant portion of healthcare providers were utilizing vitamin D for psychiatric illnesses despite lack of robust literature to support its efficacy.
Conclusion
It is unknown if vitamin D insufficiency or deficiency in psychiatric patients is due to a consequence of psychiatric disease or has a role in pathogenesis from a common pathway.[2] Due to the body’s synthesis of vitamin D from sunlight, it can be expected for an inpatient population who may be institutionalized and patients with psychiatric illness in the community who are often subject to social isolation and decreased sun exposure, to have insufficient levels.[2],[23] Patients with severe psychiatric illnesses may have a multi-drug regimen, including medications that can inhibit the body’s synthesis and absorption of vitamin D.[14]
Although recent literature supports that vitamin D has implications in the CNS, there is a lack of consistent results in human studies.[17] Specifically with psychiatric patient populations, it is hard to define a cause and effect relationship; for instance, diminished cognitive capacity or social withdrawal could result in poor diet and less outdoor activity therefor affecting vitamin D levels.[17],[25] Other confounding factors between studies include utilizing different threshold serum values, different grading scales for psychiatric symptoms, various patient sample sizes, and length of study period. Also, because there is no optimum dose or duration of vitamin D determined for neurologic diseases, supplementation, and frequency serum levels were obtained also contribute to inconsistent results.[17] It is important to consider the risk versus benefit of adding a supplement with questionable efficacy into a psychiatric medication regimen. Increased pill burden and regimen complexity increases the patient’s risk of adverse drug reactions and potential psychiatric exacerbation (e.g., patient may inadvertently take a weekly vitamin D dose daily instead of a scheduled daily antipsychotic). In addition to the risk of self administration errors, the increased medication costs creates further barriers to appropriate adherence.Vitamin D supplementation should be used in caution due to inconclusive evidence for vitamin D supplementation for treatment in psychiatric illness.
References
1. McCue R, Charles R, Orendain G, Joseph M, Abanishe J. Vitamin D Deficiency Among Psychiatric Inpatients. The Primary Care Companion for CNS Disorders. 2012;14(2).
2. Rylander M, Verhulst S. Vitamin D Insufficiency in Psychiatric Inpatients. Journal of Psychiatric Practe. 2013;19(4):296–300.
3. Graham KA, Keefe RS, Lieberman JA, Calikoglu AS, Lansing KM, Perkins DO. Relationship of low vitamin D status with positive, negative and cognitive symptom domains in people with first-episode schizophrenia. Early Intervention in Psychiatry. 2014. doi: 10.1111/eip.12122.
4. Toffanello ED, Sergi G, Veronese N, et al. Serum 25-hydroxyvitamin D and the onset of late-life depressive mood in older men and women: the Pro.V.A. study. J Gerontol A Biol Sci Med Sci. 2014;69(12):1554–1561.
5. Polak MA, Houghton LA, Reeder AI, et al. Serum 25-hydroxyvitamin D concentrations and depressive symptoms among young adult men and women. Nutrients. 2014;6(11):4720–4730.
6. Spedding S. Vitamin D and depression: a systemic review and meta-analysis comparing studies with and without biological flaws. Nutrients. 2014;6:1501–1518.
7. Kalueff AV, Tuohimaa P. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Current Opinion in Clinical Nutrition and Metabolic Care. 2007;10:12–19.
8. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. Journal of Clinical Endocrinology & Metabolism. 2011;96(7):1911–1930.
9. Bhimani MM. Vitamin D: does it play a role in psychiatry? J Pak Med Assoc. 2012;62(2):181–182.
10. Norelli LJ, Coates AD, Kovasznay BM. A comparison of 25-hydroxyvitamin D serum levels in acute and long-stay psychiatric inpatients: A preliminary investigation. e-SPEN. 2010;5(4):187–189.
11. Abdullah AK, Khan S, Mustafa SF, et al. Vitamin D status and cardiometabolic risk factors in long-term psychiatric inpatients. Prim Care Companion CNS Disord. 2012;14(1).
12. Drocourt L, Ourlin JC, Pascussi JM, et al. Expression of CYP3A4, CYP2BB6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. The Journal of Biological Chemistry. 2002;277:25125–25132.
13. Gronli O, Kvamme JM, Jorde R, Wynn R. Vitamin D deficiency is common in psychogeriatric patients, independent of diagnosis. BMC Psychiatry. 2014;14:134–140.
14. Robien K, Oppeneer SJ, Kelly JA, Hamilton-Reeves JM. Drug–Vitamin D Interactions: A Systematic Review of the Literature. Nutrition in Clinical Practice. 2013;28(2):194–208.
15. Anderson PH, May BK, Morris HA. Vitamin D Metabolism: New Concepts and Clinical Implications. The Clinical Biochemist Reviews. 2003;24(1):13–26.
16. Valipour G, Saneei P, Esmailzadeh A. Serum vitamin D levels in relation to schizophrenia: a systemic review and meta-analysis of observational studies. J Clin Endocrinol Metab. 2014;99(10):3863–3872.
17. Anastasiou CA, Yannakoulia M, Scarmeas N. Vitamin D and cognition: an update of the current evidence. Journal of Alzheimer’s Disease. 2014;42:S71–S80.
18. Yuksel RN, Altunsoy N, Tikir B, et al. Correlation between total vitamin D levels and psychotic psychopathology in patients with schizophrenia: therapeutic implications for add-on vitamin D augmentation. Ther Adv Psychopharmacol. 2014;4(6):268–275.
19. Humble M, Gustafsson S, Bejerot S. Low serum levels of 25-hydroxyvitamin D (25-OHD) among psychiatric out-patientsin Sweden: relations with season, age, ethnic origin and psychiatric diagnosis. J Steroid Biochem Mol. 2010;121(1-2):467–470.
20. Itzhaky D, Amital D, Gorden K, et al. Low serum vitamin D concentrations in patients with schizophrenia. Isr Med Assoc J. 2012;14(2):88–92.
21. Bhargava P, Cassard S, Steele SU, et al. The vitamin D to ameliorate multiple sclerosis (VIDAMS) trial: study design for a multicenter, randomized, double-blind controlled trial of vitamin D in multiple sclerosis. Contemporary Clinical Trials. 2014;39:288–293.
22. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systemic review. Journal of Clinical Endorinology and Metabolism. 2013;99(3):757–767.
23. Jamillian H, Bagherzadeh K, Nazeri Z, et al. Vitamin d, parathyroid hormone, serum calcium and phoshorus in patients with schizophrenia and major depression. Int J Psychiatry Clin Pract. 2013;17(1):30–34.
24. Valipour G, Saneei P, Esmaillzadeh A. Serum vitamin D levels in relation to schizophrenia: a systemic review and meta-analysis of observational studies. J Clin Endocrinol Metab. 2014;99(10):3863–3872.
25. Schneider B, Weber D, Frensch A, et al. Vitamin D in schizophrenia, major depression and alcholism. J Neural Transm. 2000;107(7):839–842.




