by Suzanne C. Perkins, Eric D. Finegood, and James E. Swain
by Suzanne C. Perkins, Eric D. Finegood, and James E. Swain
Dr. Perkins is from Department of Psychiatry, Faculty of Medicine, University of Michigan, Ann Arbor, Michigan; Mr. Finegood is from NYU Steinhardt School of Culture, Education, and Human Development, New York, New York; and Dr. Swain is from Department of Psychiatry, Faculty of Medicine, University of Michigan, Ann Arbor, Michigan, and Yale Child Study Center Yale, Yale University, New Haven, Connecticut.
Innov Clin Neurosci. 2013;10(4):10–19
Funding: This work was supported by grants from the NIH/NIMHD IRC2MD004767-01, National Alliance of Research on Schizophrenia and Depression (NARSAD) (bbrfoundation.org/), and University of Michigan resources including the Robert Wood Johnson Health and Society Scholars Program, the Center for Human Growth and Development, and the Michigan Institute for Clinical Health Research UL1TR000433.
Financial Disclosures: The authors do not have conflicts of interest relevant to the content of this article.
Key Words: Childhood poverty, low socioeconomic status (SES), language, parenting, brain networks, developmental neuroscience, stress, social health disparities
Abstract: Socioeconomic status affects a variety of mental and physical health outcomes, such as language development. Indeed, with poverty, disparities in the development of language processing are arguably among the most consistently found— with decreases in vocabulary, phonological awareness, and syntax at many different developmental stages. In this review, after considering basic brain systems affected by low socioeconomic status that are important for language development and related peripartum issues, we focus on two theoretical models that link poverty with the brain systems affected in language problems. The family stress model connects poverty with parental emotional distress that affects parenting, whereas the parental investment model involves a focus on basic needs that affects children’s language. Understanding the mechanisms through which poverty affects the brain, parenting behaviors and language development may have implications for identification and treatment of individuals as well as social policy.
Introduction
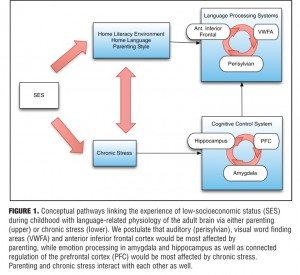
Chronic, long-term poverty or low socioeconomic status (SES) is negatively associated with a variety of mental and physical adverse health outcomes.[1] Disparities in the development of language processing are arguably among the most consistent, including vocabulary, phonological awareness, and syntax at many different stages of development,[2–4] along with memory and cognitive control mental functions.[5,6] In this review, we begin with an examination of candidate mechanisms by which low socioeconomic status influences brain development. Recent research has demonstrated effects of poverty on brain functions in language and executive function areas in particular. We also discuss possible environmental and behavioral mediators of the effects of low SES on language development through pregnancy, the perinatal period, and parenting. Next, we focus on two theoretical models that link poverty and adverse language development outcomes with consideration of related underlying brain physiology. First, we consider the family stress model, which posits that economic stress causes an increase in parental emotional distress and results in harsher, more authoritarian parenting practices and fewer opportunities for affection and nurturing.[7] Second, we review the family investment model, which suggests that families in poverty, by necessity, focus time on the acquisition and assessment of basic needs.[8] Finally, we conclude with discussions of how these models are helpful in understanding contributions of family dynamics and environment in the development of language processing and may inform the interpretation of research on biological mechanisms of poverty (Figure 1). Most likely, poverty has adverse effects on language through both effects on parenting and increased stress, suggesting opportunities for early detection and treatment.
SES, Brain, and Language
There is good evidence that low SES is a stressful condition associated with deficits in brain physiology in regions associated with typical language development. One early study9 described perisylvian deficits associated with low SES among children identified as poor readers. They found that children with poor phonemic awareness skills, despite higher SES backgrounds, had increased perisylvian function during a reading task. This was not the case for children with both low SES and low phonemic awareness. Moreover, low SES has been positively correlated with the degree to which left (relative to right) inferior frontal gyrus is activated during a language task in young children, indicating decreased specialization of language function in the left hemisphere in children with low SES.[10] These studies suggest that social, cognitive and underlying neurobiological influences on reading development are fundamentally related.[9,10]
Poverty is also associated with deficits in the psychological underpinnings of language learning, which are keenly dependent on executive functioning and memory—both of which are vulnerable to stress.[6,10] Consistent with a high stress level, low SES children have higher levels of salivary cortisol,[11] which may explain some of the functional and structural findings[12] in brain areas that regulate stress hormones including the hippocampus (HC), amygdala (AG), and prefrontal cortex (PFC),[13]—all areas that are also important for aspects of executive function and memory. The related measure of perceived stress in adulthood has also been associated with decreased HC grey matter volume,[14] and low SES is associated with increased activity in the AG[15] suggesting a mechanism where increased glucocorticoids, as a result of the stress of poverty, leads to decreased HC volume and increased AG activity. This dysregulation of stress response in educational settings likely interferes with the acquisition of language both directly by distraction and through adverse effects on executive function development.[16,17] There is a current controversy over whether specific language impairments may result from impairment to a “domain-specific” system devoted to language itself or from some more “domain-general” system,[18] but this has not yet been studied among low SES children. However, one potential hypothesis is that since attention is subserved by the PFC,[19] likely any dysfunction in this region would also interfere with the development of language. Finally, adults with lower subjective social status have reduced grey matter volume in the executive function regions of the perigenual anterior cingulate cortex (ACC) area of the medial PFC.[20] Thus, three neuroanatomical areas, AG, HC and PFC, work together to regulate emotion, in what behaviorally is often termed self-control, an integral part of healthy language development,[21] and all are vulnerable to the effects of low SES.[12] Critical developmental windows of relative susceptibility to SES-related effects on the brain are likely important for language.
In a recent study on the long-term effects of childhood SES, Gianaros et al[22] found that parental education was positively associated with corticostriatal activation and connectivity. During processing of stimuli that signal monetary gains, and after controlling for own education, lower parental education predicted reduced activation in ACC and dmPFC, plus reduced connectivity between these cortical regions and OFC and striatum—established as reward and impulse-control circuits. Important to language, functional connections were also reported with anterior inferior frontal (IFG) areas within the operculum and near the Broca’s area—regions well associated with articulation and word analysis.[21] Thus, childhood poverty may be associated with impaired connectivity of language areas with reward and impulse-control.[22]
Indeed, the association between SES and language may be stronger than for other neurocognitive systems,[6,10] as it accounts for almost a standard deviation of difference between groups of high and low SES. These magnitudes of language delay can reach clinical significance, such as language impairment (LI) and speech impairment (SI). LI and SI are often thought of as being more genetic than environmental; however, there is mounting evidence that SES plays an important role in the development of LI and SI. In one study, children with LI had younger mothers with both lower levels of education and income22—factors that influenced the growth rate of vocabulary throughout childhood. In fact, for every gain of $5,000 in annual income, vocabulary scores were raised by almost two points. This effect appears to be internationally consistent. In one Chinese study,[3] SES explains five percent of variance in child vocabulary, a large effect that has important implications for long-term learning, education and potential for learning.[23]
As further evidence of the importance of LI to child development, a long-term study of children with SI and LI[24] identified in early childhood found that LI was related to the worst outcomes in adulthood including academic achievement. In this study, children with LI and/or SI had significantly lower SES and intelligence quotient (IQ), which contributed unique variance to specific aspects of achievement. When controlling for these factors, children with SI largely remediated deficits by early adulthood; however, children with LI continued to show deficits in memory and executive function at age 19. In addition, children with LI at age 5 had a 3- to 10-fold increased likelihood of spelling, math, and reading disabilities by age 19.[24] LI is also associated with long-term consequences for mental health, including attention deficit hyperactivity disorder (ADHD); affective and anxiety disorders, particularly social phobia and generalized anxiety disorder;[25] somatic complaints and delinquency;[26] high school dropout and adult low income;[27] and suicide.[28] LI is a profound problem for which more research is required to elucidate the biological and environmental mechanisms at work, especially among children born into poverty where those risks are higher due to inadequate nutrition, unstable living conditions, and/or poor quality medical care. For example, issues such as chronic ear infections may not be adequately managed and the associated symptoms could diminish concentration and interfere with the child’s ability to effectively use auditory processing to discriminate speech sounds.
Pregnancy, Preterm Birth, and the Perinatal Period
Low SES environments may affect language development through pregnancy and the early postpartum. Prematurity, defined as less than 37 weeks of gestation, is more common in high poverty contexts[29] and may contribute to the development of language delays through maternal stress effects on the fetus.[30] Infants born prematurely have lower birth weights, a measure associated with a range of brain differences compared to full-term babies, which may help explain language effects, such as decreased grey matter volumes and levels of myelinated white matter.[31]
Indeed, brain volume differences are associated with mental status as early as two weeks postpartum.[32] A large literature on fetal programming has shown that low SES and psychosocial stress adversely affects fetal development.[33–35] Finally, maternal stress is related to lower birth weight,36 which is also associated with developmental delays in brain development associated with language.
In accord, preterm infants show delays in general cognition and both receptive and expressive language.[37] In one study,[38] preterm infants had delays in both receptive and expressive language at 26 months (corrected age) of between 3 and 5 months. Language delays were also correlated with both length of hospital stay after birth (receptive) and APGAR score (appearance, pulse, grimace, activity, respiration) at birth (expressive). Also, receptive language was positively associated with birth weight suggesting that developmental stage at birth may be an important factor in long-term development of language. Importantly, maternal behavioral sensitivity was associated with better receptive language skills suggesting a buffering effect of the mother, offering protection from environmental factors.
Environmental risk and resilience factors related to poverty are important. Elevated risk levels can be unexpectedly high anywhere leading to perinatal stress and adverse language development; and resilience factors may suggest effective interventions. In a fascinating natural experiment, Laplante et al[39] found that for infants who were in utero during an ice storm in Canada where families lived without electricity for several days, levels of prenatal maternal stress during the storm were related to communication development in the infants at two years old. In a follow-up study using a measure of the degree of exposure to actual threat, loss and change related to the event, the researchers found that prenatal stress was associated with child lower IQ and vocabulary at age five.[40] There is some evidence that as children age, stress during pregnancy has less of an effect on language ability. In a large cohort study, Whitehouse et al[41] found that high levels of stress in early pregnancy had no effect on children’s language at age 10 but stress during late pregnancy lowered language ability at a trend level. This research highlights the importance of the mother-infant relationship in supporting child language—perhaps especially in the face of psychosocial stress.
Parenting Style
Parent-child interactions have an undeniable influence on developing children at every socioeconomic level. A parenting style that includes parental warmth combined with high expectations and clear rules and routines moderates other negative effects of poverty, and lack of parental warmth may be implicated in the development of language problems. Parent warmth is associated with numerous positive outcomes in later childhood and adulthood including better memory,[23] higher achievement, language ability, and income.[42] When mother-infant interactions are less positive, however, receptive and expressive language can be compromised.[37] In a study of mother-infant dyadic interactions, mothers with the highest amount of negative control strategies had children who used the shortest sentences and fewest grammatical word types and number of different word roots.[43] Conversely, maternal sensitivity and communication with the child around cognitive states (such as asking the child “Is this hard for you?”) is supportive of child executive functioning and self-regulation,[44] suggesting that this high-quality, early parent-child interaction is important for the development of underlying skills necessary for language development. Furthermore, in a large study of mother-child video-taped interactions, both income and maternal education predicted parenting quality for children at one, two, and three years of age, such that parents with higher SES had higher sensitivity and positive regard, showed more evidence of teaching during a teaching task, and were rated higher on supportive presence and quality of assistance on a complex puzzle task.[45] Parenting quality then was a function of SES and predicted mental health development in each age group.
Key brain structures and circuits are emerging as plausible mediators of the effects of low SES, peripartum stress, and low quality parenting. For example, adults who experienced adverse parenting during childhood have smaller hippocampus volumes in adulthood.[46,47] In one pioneering neuroimaging study, it was found that mothers who reported experiencing higher maternal care in their own childhood showed increased gray-matter volumes in frontal executive control brain regions, and more brain activity in response to baby-cry versus control sound in executive control regions and the hippocampus.[48] This suggests a long-term impact of parenting on brain structure and function in regions that are related to SES-effects and language.
Another important predictor of parenting quality was maternal reading frequency, suggesting that parental literacy, independent of education level, may mediate the effects of SES in the development of maternal-infant bonds. Intervention studies have also found preliminary evidence that stimulation, in the form of visits focused on parent-child interactions, can reduce mental health problems in adolescence in accord with the idea that the effects of parenting style are truly long lasting.[49] Also in accord, exposure to parental corporal punishment in childhood is associated with reduced grey matter volume in medial PFC, dorsolateral-PFC, and ACC.[50]
One related study of the United States African American-Caucasian achievement gap found that low SES was related to degree of adolescent autonomy, parental monitoring, school orientation and warmth.[51] Both a higher level of autonomy and higher school orientation were related to positive school outcomes, overall suggesting that these parental styles may moderate achievement. There are putative brain circuits that moderate the direct effects of harsh parenting on the amygdala and PFC.[52] This is supported by studies of adults with adverse, early childhood family environments that show high AG reactivity during an emotion labeling task, which were positively correlated with high right ventrolateral prefrontal cortex (rvlPFC) activity. This can be potentially interpreted as decreased ability of rvlPFC to modulate AG activation.
Poverty is thus associated with lower parental quality as measured by warmth, autonomy, and monitoring through stressors that center around food, job, and housing insecurity.[7] Maternal stress has been shown to be transmittable to children and to negatively influence infants and small children. In one study of mother-infant dyads, high maternal stress was associated with distress at six months, as well as anger and deficits in attention at five years old.[53] In the Magill-Evans study,[37] both mother-spouse and mother-child relationships were influential in language development. Low rating of the spousal relationship and higher rating of stress related to the child’s distractibility both negatively influenced children’s receptive language, suggesting that multiple sources of stress in the home influence language and cognition.
Language Use and the Home Literacy Environment
Stress has varied effects beyond the direct effects of the biological mechanisms of stress on development. Stress is also linked to communication and language development.[39,40] For example, parental use of language and evidence of reading as a leisure time activity predicts cognitive and language development. In Hoff’s study of children in China,[3] SES was predictive of five percent of language development, but because SES was related to maternal vocabulary, maternal vocabulary completely mediated the relation between SES and language, underlining the importance of parent literacy and suggesting that families in poverty where parents use complex and varied language buffer their children and support normative or advanced language development in children.
Furthermore, Hart and Risley[54] conducted a particularly influential study on parental education and word usage in the home. In their study, total number of words spoken in the home varied greatly between families, and word usage was the single strongest determinant of child vocabulary growth. Most importantly for the relation between poverty and vocabulary growth, less educated parents have been shown to be likely to use fewer words, less complicated syntax, and fewer references to events not in the present when communicating with their children.[54] Low language complexity in lower income homes was also a major predictor of vocabulary growth in children. This relation has been repeatedly replicated in other studies. Most recently, Huttenlocher[55] studied the role of caregiver speech by videotaping 47 parent-child dyads and examining speech patterns. Language usage is a complex idea, consisting of multiple aspects including quality and diversity of lexical utterances and constituent and clausal diversity. Lexical diversity is the number of different word employed by both children and caregivers. Constituent diversity includes the inclusion of optional words in a clause including adjectives, adverbs, qualifiers, and possessive. For example “pick up the ball” differs from “Can you please pick up your red, striped ball quickly and bring it over to me?” in the number of optional words used. Finally clausal diversity is the use of different ways of combining clauses, including combining two clauses and using modifying clauses. Parent language usage remains relatively constant over this time, with the exception of greater clausal diversity by parents as children age.55 However, children develop new language exponentially between 14 and 46 months with a curvilinear increase in both lexical and constituent diversity and linear increase in clausal diversity. By 14 months, there are remarkable differences in the complexity and variety of child language, and these differences in language ability tend to remain over time. At 26 months, there are already significant differences in both the number of word types and the constituent diversity, with the highest rung of SES approximately double the initial starting point of the lowest SES children. Since these differences remain relatively stable, this achievement gap remains throughout the first four years studied. For clausal diversity, Huttenlocher[55] found a linear increase in diversity with SES. This may be in part because initial clausal diversity in any SES group is basically nonexistent, suggesting that the difference in clausal diversity found at four years of age may remain over time.
Importantly, there is a high degree of predictive value of caregiver speech patterns to child speech patterns in all three areas. Variability in caregiver speech partially mediated the relation between SES and lexical diversity and fully mediated the relation with constituent diversity but did not influence relation between SES and clausal diversity. This suggests that greater parent language usage and diversity can influence language development positively, regardless of SES status. In fact, in children with early brain injuries, parent language, as measured by mean length of utterances, is related to a higher vocabulary growth rate than in typically developing children,[56] underlining the importance of parental language usage in mediating the effects of early adversity.
Although many studies of parent-child interactions focus on the mother-child relationship, there is evidence that father’s language usage is also important in child language development. In one study, fathers’ vocabulary was additive to mother characteristics in predicting child language development.[57] Nonverbal gestures are influential in the development of language in early childhood as well. In one study of gesture and word types in parent-child interactions, SES was related to the number of gestures used by parents and paternally mediated vocabulary usage at school age.[58]
There is evidence that aspects of the environmental stress of poverty may negatively influence parental language usage. In a reanalysis of the Hart and Risley data, Evans et al[59] studied the role of crowding within the home on parental language.
Crowding in the home is a measure of stress in that more people in the home increases noise density and minimizes opportunities for mental quiet. SES was predictive of greater crowding in the home and of less language diversity; however, density independently predicted less diversity of language, controlling for SES. Again, parental responsiveness, controlling for SES, mediated that role of crowding but SES remained a significant predictor of language density.
Parental style, which is an important predictor of language development in children, is also important for understanding patterns of word usage in the home. In the Taylor study,[43] high guidance mothers (mothers whose control strategies focused on guiding the child to help the child comply with directions) had the longest mean length of utterance and highest number of different word roots, and this was, in turn, associated with greater complexity in child language development.
Parent language environment is likely associated with the child’s development of brain regions associated with language reception and expression; however, there are no studies, to our knowledge, directly addressing this association. There is a recent study[60] that suggests that in children with higher parental language usage have better novel rule learning skills (the ability to learn a new rule related to a previously learned rule) and use a region of the PFC, the middle frontal gyrus (MFG, an area associated with rule learning), less, suggesting that they need less activity in this region during rule learning. The same pattern was exhibited in relation to SES. Comparing high and low SES children, a higher income-to-needs ratio was related to greater accuracy in new rule learning and less use of the MFG.[60]
One focus of intervention in language development has been the home literacy environment (HLE), which has been characterized by a number of researchers as influential in the development of child language.[23,61,62] Birth to Three programs that give families books at pediatric visits and encourage parents to read to children (e.g., Reach Out and Read[63]) or that encourage parent-child reading are centered on the idea that language in the home is one of the key areas where social programs can influence child language development. High SES homes have rich home literacy environments (HLE) and the reverse is true for low SES homes. Parental SES, parental reading, and language behaviors are significantly associated with the richness of the HLE, often measured using the HOME (Home Observation for the Measurement of the Environment) inventory.[64] The HOME inventory investigates materials and practices in the home related to literacy, including emotional and verbal responsivity, and includes acceptance of the child, organization of the environment, provision of appropriate play materials, and maternal involvement with the child under a variety of daily situations.
Stable rich HLEs have the greatest effect on kindergarten literacy skills.[65] In fact, rich HLE in early childhood that later decline are not as protective as those that remain rich over time. However, even moderately rich HLEs are better than impoverished environments for language function measured at kindergarten. These effects are long lasting. HLE predicts language at three years old, which predicts language at 4.5, and, perhaps most importantly, changes in the HLE between three and 4.5 predict language at 4.5 even after controlling for current HLE.[66] In one study,[62] early HLE influenced kindergarteners’ vocabulary and conceptual knowledge, which, in turn, predicted printed word recognition, a precursor to strong reading. These kindergarten pre-reading skills predicted reading skills into the second grade. In another study,[23] scores on the environmental stimulation subscale of the HOME predicted language ability in middle school. The HLE is not only important for language but is important in the development of cognitive skills that underlie the development of language, such as self-regulation.[66]
The role of mother-child interactions is important in the HLE too. In the Sarsour study,[66] maternal depression was negatively associated with change in the HLE, underlining the importance of maternal mental health in child development. However, maternal education and number of hours worked predicted higher change in HLE, suggesting that maternal education and financial independence are important to the development of HLE. Although there is little evidence in human literature of specific associations between an enhanced HLE and the development of certain brain regions, there is a literature on the role of environmental deprivation on the brain development. Environment deprivation is associated with deficits in IQ and cognitive development,[67] as well as expressive and receptive language.[68] In these natural experiment studies, children reared in large institutions and adopted or brought into enhanced foster care were examined to determine the role of environmental deprivation in cognitive and brain development. Behaviorally, children placed in enhanced foster care before age two had good cognitive development and IQ outcomes[67] and improved expressive and receptive language.[68]
In terms of brain functioning, early socio-emotional, including linguistic, deprivation is associated with deficits in the white matter fiber tract connecting the PFC to limbic structures, the uncinate fasciculus.[69] In these institutionalized groups, there are also documented deficits in the amygdala.[70] In a study using electroencephalogram (EEG), children who were institutionalized had brain activity consistent with younger children and those placed in enhanced foster care, prior to age two, showed similar brain patterns to children that were never institutionalized, suggesting that it is possible to remediate even serious social deprivation with early intervention.[71] The brain deficits, located in the PFC and temporal regions, mediate the relationship between institutional deprivation and self-control, a precursor to strong language skills.[72] Importantly institutionalized children exhibit a delay in the developmental shift in early childhood from right to left lateralization.[73] Considering the importance of left frontal and temporal regions in language development, a delay in this functional shift, may be very important to the development of language.
Discussion
The literature reviewed suggests six neuroanatomical regions as central to the development of language (Figure 1). The first three are language areas specific to auditory processing, visual processing, and word analysis and articulation, namely the perisylvian region of visual word form area (VWFA), and the anterior inferior frontal areas, respectively. We postulate that these would be most affected by environmental influences such as parenting. In addition, areas associated with emotional processing, namely the amygdala and hippocampus, as well as PFC regions associated with cognitive control over emotion are central to self-regulation and its role in the development of language ability and may be especially vulnerable to the effects of chronic stress.
Parenting styles that include warmth, promotion of autonomy, and encouragement are predictive of strong language skills37 and better self-regulation,[44] while controlling parenting strategies relate to the opposite.[43] The ability to self-regulate in a learning situation is related to both SES and to parental language through the PFC.[60]
Research implications. There are several converging lines connecting poverty to language problems through stress, home language use, home literacy environment, and self-regulation, yet many questions remain unanswered. Although poverty is related to language development through a number of pathways, in many studies, when controlling for other factors, poverty is still predictive of deviations from normative language development. Among unanswered questions is that if poverty has a unique effect on language development, what is the mechanism through which poverty, controlling for other factors, influences language? One possible mechanism is the direct effects of stress on language development. Another mechanism involves perinatal and parenting factors (Figure 1). Studies aimed at understanding the basic mechanisms of the perinatal period will help to understand the role of maternal stress in brain development supporting language.
The literature on environmental deprivation has demonstrated delays in left-right lateralization, development of the PFC,[72] increased amygdala,[70] and white matter disruption linking these regions.[69] Taken together, these suggest a neuroanatomical pathway for decreased cognitive control, necessary for the higher order planning needed for language development17 and for understanding content.[74]
Although a growing number of studies have begun to explore the neurohormonal basis of parenting thoughts and actions in humans,[75–77] it remains largely unknown how socioeconomic factors, either growing up in poverty or experiencing poverty later in life as an adult, affects the human parental brain—that is, specific brain circuitry that promote parental thoughts and behaviors. Studies of this kind are still necessary to understand the risks associated with low SES and brain areas involved in caregiving motivation, which potentially simultaneously regulate approach and avoidance motivations to infant cues and are tied to the regulation stress response.[78,79] These brain areas and caregiving behaviors are perhaps critical to creating the nurturing environment necessary for normal language development in early life.
A bright spot is that parental language use, independent of SES, is predictive of the development of normative language. This is an important area where there has been limited previous research. Using the HOME interview, several researchers have found evidence that increases in the HLE are predictive of better language skills but studies that directly link HLE enhancement to neural function are lacking. It is an open question whether there are both language deficits and perisylvian brain region deficits in low SES and whether they still occur after controlling for language ability in adults.
Finally, we present a caveat in this area of research given the focus on parenting and the home environment. Children from low SES backgrounds may have school circumstances that contribute to language development problems. Such children often attend schools that have high student-to-teacher ratios, provide poor quality instruction, and lack access to academic resources (e.g., textbooks) that foster language development. Therefore, we acknowledge that the school environment deserves attention in models of language development and SES effects. Indeed, this may be an area of fruitful research in which certain improvements in school environments may compensate for SES-related problems.
Policy implications. For each $5,000 in extra income annually, vocabulary is raised an average of 2 points on a standard scale vocabulary measurement.3 Given that language deficits last into early adulthood[24] and language delays increase the risk of long-term mental health outcomes,[80] understanding the underlying neural mechanisms behind language delays in high poverty contexts has important public health implications. Importantly the number of words spoken in the home can increase children’s vocabulary by 300 words at age two.[80] This provides strong evidence that intervention in maternal-infant interactions, prior to age two, will have long-lasting effects on child language development and consequently on long-term health and welfare.
References
1. Singh-Manoux A. Socioeconomic trajectories across the life course and health outcomes in midlife: evidence for the accumulation hypothesis? Int J Epidemiol. 2004;33:1072–1079.
2. Whitehurst GJ. Language processing in context: language learning in children reared in poverty. In: Adamson LB, Romsky MA (eds). Research on Communication and Language Disorders: Contribution to Theories of Language Development. Baltimore, MD: Brookes; 1997:233–266.
3. Hoff E, Tian C. Socioeconomic status and cultural influences on language. J Commun Disord. 2005;38:271–278.
4. Noble K, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev Sci. 2007;10:464.
5. Evans GW. Child development and the physical environment. Annu Rev Psychol. 2006;57:423–451.
6. Farah MJ, Shera DM, Savage JH, et al. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110:166–174.
7. McLoyd VC. The impact of economic hardship on black families and children: psychological distress, parenting, and socioemotional development. Child Dev. 1990;61:311–346.
8. Conger RD, Donnellan MB. An interactionist perspective on the socioeconomic context of human development. Annu Rev Psychol. 2007;58:175–199.
9. Noble KG, Wolmetz ME, Ochs LG, et al. Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Dev Sci. 2006;9:642–654.
10. Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev Sci. 2005;8:74–87.
11. Lupien S, King S, Meaney M, McEwen B. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:653–676.
12. Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11:651–659.
13. Pruessner JC, Dedovic K, Pruessner M, et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations. Psychoneuroendocrinology. 2009:1–13.
14. Gianaros PJ, Jennings JR, Sheu LK, et al. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35:795–803.
15. Gianaros PJ, Horenstein JA, Hariri AR, et al. Potential neural embedding of parental social standing. Soc Cogn Affect Neurosci. 2008;3:91–96.
16. Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Dev. 2007;78:647–663.
17. Im-Bolter N, Johnson J, Pascual-Leone J. Processing limitations in children with specific language impairment: the role of executive function. Child Dev. 2006;77:1822–1841.
18. van der Lely HK. Domain-specific cognitive systems: insight from Grammatical-SLI. Trends Cogn Sci. 2005;9:53–59.
19. Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140.
20. Gianaros PJ, Horenstein JA, Cohen S, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci. 2007;2:161–173.
21. Shaywitz S, Shaywitz B. Paying attention to reading: the neurobiology of reading and dyslexia. Dev Psychopathol. 2008;20:1329–1349.
22. Gianaros PJ, Manuck SB, Sheu LK, et al. Parental education predicts corticostriatal functionality in adulthood. Cereb Cortex. 2011;21:896–910.
23. Farah MJ, Betancourt L, Shera DM, et al. Environmental stimulation, parental nurturance and cognitive development in humans. Dev Sci. 2008;11:793–801.
24. Young A, Beitchman JH, Johnson C, et al. Young adult academic outcomes in a longitudinal sample of early identified language impaired and control children. J Child Psychol Psychiatry. 2002;43:635–645.
25. Goldston DB, Walsh A, Mayfield Arnold E, et al. Reading problems, psychiatric disorders, and functional impairment from mid- to late adolescence. J Am Acad Child Adolesc Psychiatry. 2007;46:25–32.
26. Arnold EM, Goldston DB, Walsh AK, et al. Severity of emotional and behavioral problems among poor and typical readers. J Abnorm Child Psychol. 2005;33:205–217.
27. Johnson C, Beitchman JH, Brownlie EB. Twenty-year follow-up of children with and without speech-language impairments: family, educational, occupational, and quality of life outcomes. Am J Speech-Language Pathol. 2010;19:51.
28. Daniel SS, Walsh AK, Goldston DB, et al. Suicidality, school dropout, and reading problems among adolescents. J Learn Disabil. 2006;39:507–514.
29. DeFranco EA, Lian M, Muglia LA, Schootman M. Area-level poverty and preterm birth risk: a population-based multilevel analysis. BMC Public Health. 2008;8:316.
30. Lou HC, Hansen D, Nordentoft M, et al. Prenatal stressors of human life affect fetal brain development. Dev Med Child Neurol. 1994;36:826–832.
31. Hüppi PS, Warfield S, Kikinis R, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43:224–235.
32. Peterson BS, Anderson AW, Ehrenkranz R, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111:939–948.
33. Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr Opin Endocrinol Diabet Obes. 2010;17:507–516.
34. Wadhwa PD, Entringer S, Buss C, Lu MC. The contribution of maternal stress to preterm birth: issues and considerations. Clin Perinatol. 2011;38:351–84.
35. Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289.
36. Wadhwa PD, Sandman CA, Porto M, et al. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstetr Gynecol. 1993;169:858–865.
37. Magill-Evans J, Harrison MJ, Van der Zalm J, Holdgrafer G. Cognitive and language development of healthy preterm infants at 10 years of age. Phys Occup Ther Pediatr. 2002;22:41–56.
38. Cusson RM. Factors influencing language development in preterm infants. J Obstet Gynecol Neonat Nurs. 2003;32:402–409.
39. Laplante DP, Barr RG, Brunet A, et al. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr Res. 2004;56:400–410.
40. Laplante DP, Brunet A, Schmitz N, et al. Project Ice Storm: prenatal maternal stress affects cognitive and linguistic functioning in 5½-year-old children. J Am Acad Child Adolesc Psychiatry. 2008;47:1063–1072.
41. Whitehouse AJO, Robinson M, Zubrick SR, et al. Maternal life events during pregnancy and offspring language ability in middle childhood: the Western Australian Pregnancy Cohort Study. Early Hum Dev. 2010;86:487–492.
42. Singh-Manoux A, Fonagy P, Marmot M. The relationship between parenting dimensions and adult achievement: evidence from the Whitehall II Study. Int J Behav Med. 2006;13:320–329.
43. Taylor N, Donovan W, Miles S, Leavitt L. Maternal control strategies, maternal language usage and children’s language usage at two years. J Child Lang. 2009;36:381–404.
44. Bernier A, Carlson SM, Whipple N. From external regulation to self-regulation: early parenting precursors of young children’s executive functioning. Child Dev. 2010;81:326–339.
45. Lugo-Gil J, Tamis-LeMonda CS. Family resources and parenting quality: links to children’s cognitive development across the first 3 years. Child Dev. 2008;79:1065–1085.
46. Buss C, Lord C, Wadiwalla M, et al. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci. 2007;27:2592–2595.
47. Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18:729–736.
48. Kim P, Leckman JF, Mayes LC, et al. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav Neurosci. 2010;124:695–700.
49. Walker SP, Chang SM, Powell CA, et al. Effects of psychosocial stimulation and dietary supplementation in early childhood on psychosocial functioning in late adolescence: follow-up of randomised controlled trial. BMJ 2006;333:472–474.
50. Tomoda A, Suzuki H, Rabi K, et al. Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage. 2009;47:T66–T71.
51. Mandara J, Varner F, Greene N, Richman S. Intergenerational family predictors of the black–white achievement gap. J Educat Psychol. 2009;101:867–878.
52. Taylor SE, Eisenberger NI, Saxbe D, et al. Neural responses to emotional stimuli are associated with childhood family stress. Biol Psychiatry. 2006;60:296–301.
53. Pesonen A, Raikkonen K, Heinonen K, et al. A transactional model of temperamental development: evidence of a relationship between child temperament and maternal stress over five years. Soc Dev. 2008;17:326.
54. Hart B, Risley TR. Meaningful Differences in the Everyday Experience of Young American Children. Baltimore, MD: Paul H Brookes Pub Co; 1995.
55. Huttenlocher J, Waterfall H, Vasilyeva M, et al. Sources of variability in children’s language growth. Cogn Psychol. 2010;61:343–365.
56. Rowe ML, Levine SC, Fisher JA, Goldin-Meadow S. Does linguistic input play the same role in language learning for children with and without early brain injury? Dev Psychol. 2009;45:90–102.
57. Pancsofar N, Vernon-Feagans L. Fathers’ early contributions to children’s language development in families from low-income rural communities. Early Child Res Q. 2010;25:450–463.
58. Rowe ML, Goldin-Meadow S. Differences in early gesture explain SES disparities in child vocabulary size at school entry. Science (New York, NY). 2009;323:951–953.
59. Evans GW, Maxwell LE, Hart B. Parental language and verbal responsiveness to children in crowded homes. Dev Psychol. 1999;35:1020–1023.
60. Sheridan MA, Sarsour K, Jutte D, et al. The impact of social disparity on prefrontal function in childhood. PLoS One. 2012;7:e35744.
61. Son S-H, Morrison FJ. The nature and impact of changes in home learning environment on development of language and academic skills in preschool children. Dev Psychol. 2010;46:1103–1118.
62. Storch S, Whitehurst GJ. The Role of family and home in the literacy development of children from low?income backgrounds. New Direc Child Adolesc Dev. 2001;2001:53–72.
63. Zuckerman B. Promoting early literacy in pediatric practice: twenty years of Reach Out and Read. Pediatrics. 2009;124:1660–1665.
64. Bradley RH, Caldwell BM. The HOME Inventory and family demographics. Dev Psychol. 1984;20:315.
65. Rodriguez ET, Tamis-LeMonda CS. Trajectories of the home learning environment across the first 5 years: associations with children’s vocabulary and literacy skills at prekindergarten. Child Dev. 2011;82:1058–1075.
66. Sarsour K, Sheridan M, Jutte D, et al. Family socioeconomic status and child executive functions: the roles of language, home environment, and single parenthood. J Int Neuropsycholog Soc. 2011;17:120–132.
67. Nelson CA, Zeanah CH, Fox NA, et al. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science (New York, NY). 2007;318:1937–1940.
68. Windsor J, Benigno JP, Wing CA, et al. Effect of foster care on young children’s language learning. Child Dev. 2011;82:1040–1046.
69. Eluvathingal TJ. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117:2093–100.
70. Tottenham N, Hare TA, Quinn BT, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61.
71. Marshall PJ, Fox NA, Group BEIPC. A comparison of the electroencephalogram between institutionalized and community children in Romania. J Cogn Neurosci. 2004;16:1327–1338.
72. McLaughlin KA, Fox NA, Zeanah CH, et al. Delayed maturation in brain electrical activity partially explains the association between early environmental deprivation and symptoms of attention-deficit/hyperactivity sisorder. BPS. 2010;68:329–336.
73. McLaughlin KA, Fox NA, Zeanah CH, Nelson CA. Adverse rearing environments and neural development in children: the development of frontal electroencephalogram asymmetry. BPS. 2011;70:1008–1015.
74. Turkeltaub PE, Gareau L, Flowers DL, et al. Development of neural mechanisms for reading. Nature Neurosci. 2003;6:767–773.
75. Swain JE. The human parental brain: in vivo neuroimaging. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1242–1254.
76. Swain JE. Baby stimuli and the parent brain: functional neuroimaging of the neural substrates of parent-infant attachment. Psychiatry (Edgmont) 2008;5:28–36.
77. Swain JE, Kim P, Ho SS. Neuroendocrinology of parental response to baby-cry. J Neuroendocrinol. 2011;23:1036–1041.
78. Brown SL, Brown RM, Preston SD. The human caregiving aystem: a neuroscience model of compassionate motivation and behavior. In: Brown SL, Brown RM, Penner L (eds). Moving Beyond Self Interest: Perspectives from Evolutionary Biology, Neuroscience, and the Social Sciences. New York: Oxford University Press; 2012:75–88.
79. Swain JE, Konrath S, Brown SL, et al. Parenting and beyond: common neurocircuits underlying parental and altruistic caregiving. Parent Sci Pract. 2012;12:115–123.
80. Huttenlocher J, Haight W, Bryk A, et al. Early vocabulary growth: relation to language input and gender. Dev Psychol. 1991;27.