 by Farid Talih, MD, and Jean Ajaltouni, MD
by Farid Talih, MD, and Jean Ajaltouni, MD
Dr. Talih is an assistant professor and Dr. Ajaltouni is a research fellow at the American University of Beirut Medical Center Department of Psychiatry
Innov Clin Neurosci. 2015;12(11–12):21–25.
Funding: No funding was received for the preparation of this manuscript.
Financial Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Key words: Nootropics, supplements, substance abuse, cognitive enhancers, substance misuse, psychiatric adverse effects
Abstract
The misuse of nootropics—any substance that may alter, improve, or augment cognitive performance, mainly through the stimulation or inhibition of certain neurotransmitters—may potentially be dangerous and deleterious to the human brain, and certain individuals with a history of mental or substance use disorders might be particularly vulnerable to their adverse effects. We describe four cases of probable nootropic-induced psychiatric adverse effects to illustrate this theory. To the best of our knowledge this has not been previously reported in the formal medical literature. We briefly describe the most common classes of nootropics, including their postulated or proven methods of actions, their desired effects, and their adverse side effects, and provide a brief discussion of the cases. Our objective is to raise awareness among physicians in general and psychiatrists and addiction specialists in particular of the potentially dangerous phenomenon of unsupervised nootropic use among young adults who may be especially vulnerable to nootropics’ negative effects.
Introduction
Humans have historically sought to enhance and improve their mental and cognitive abilities. Chemically augmenting the human brain is the basis of nootropic brain enhancement—the development and experimentation with substances that can presumably improve cognition. A nootropic agent is any substance that may alter, improve, or augment cognitive performance, mainly through the stimulation or inhibition of certain neurotransmitters.[1] Nootropics have been shown to increase concentration and memory potential and potentiate cognitive functioning.[2,3] Nootropics include a wide range of substances, and the overall mechanisms of action for most nootropics have not been well elucidated. In this report, we describe the most commonly consumed and easily available nootropics. We also describe four cases of nootropic use that potentially led to unwanted serious adverse psychiatric effects. To the best of our knowledge this has not been previously reported in the formal medical literature.
Commonly used nootropics
The overall evidence regarding the benefits of nootropics in healthy individuals seeking mental enhancement is still controversial. Additionally, it is important to note that nootropics are not free of adverse effects. Table 1 summarizes the mechanisms of action, desired neuropsychiatric effects, and adverse effects of the common classes of nootropics detailed below.
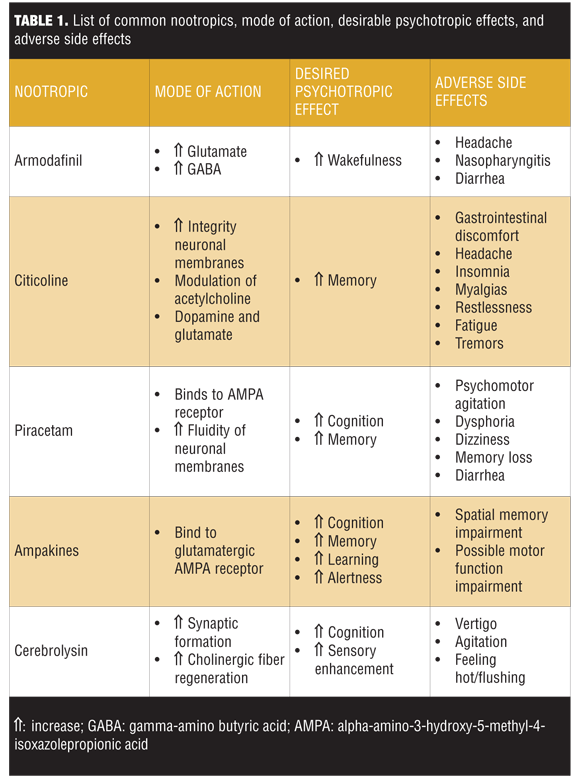
Armodafinil. Armodafinil, a wakefulness-promoting drug, is the R- isomer of racemic modafinil. Modafinil is a compound that produces an overall neuroexcitatory effect. The postulated mechanism of action is increasing the concentration of glutamate and decreasing gamma-amino butyric acid (GABA) within the posterior hypothalamus.[4] Armodafinil has been shown to improve wakefulness in patients with excessive sleepiness associated with treated obstructive sleep apnea and narcolepsy. Despite being the R-isomer of modafinil, armodafinil has a different pharmacokinetic profile and may result in improved wakefulness throughout the day compared with modafinil.[5]
Adverse effects. Despite improving wakefulness, armodafinil’s adverse effects commonly include headache, nasopharyngitis, and diarrhea.[6]
Citicoline. Citicoline, originally studied for its neuroprotective action against stroke and dementia, modulates acetylcholine, dopamine, and glutamate. It is also involved in phospholipid metabolism and enhances the integrity of neuronal membranes.[7] Citicoline has been shown to improve memory in patients with dementia as well as reduce damage to the brain after traumatic brain injury[8] or stroke.[9]
Adverse effects. Citicoline has been found to cause gastrointestinal discomfort, headache, insomnia, myalgias, restlessness, fatigue, and tremors.[10]
Piracetam. Piracetam, which is often used in early stages of Alzheimer’s disease and aging-related memory impairment,[11] is technically derived from GABA but is functionally unrelated to this neurotransmitter. It can act on the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor as an allosteric modulator binding in six different positions[12] and may have an effect on N-methyl- D-aspartate receptor (NMDA) and glutamate receptors.[13] Piracetam currently can be purchased online and is generally used for cognitive enhancement and memory improvement.[14] Piracetam has also been found to play a role in restoring membrane fluidity contributing to enhanced neuroplasticity15 and neuroprotective effects.[16]
Adverse effects. Piracetam users have reported symptoms of psychomotor agitation, dysphoria, tiredness, dizziness, memory loss, headache, and diarrhea. Many users reported to have neither felt any cognitive improvement nor psychedelic effects after taking piracetam.[17–19]
Ampakines. Ampakines are a class of drugs that bind to the glutamatergic AMPA receptor, enhancing its activity[20] and potentially triggering the induction of long-term potentiation and improvement of learning, cognition, and alertness.
Adverse effects. Ampakines have also been found to cause headaches, somnolence, and nausea.[21] Despite the enhancement of long-term cortical neural potentiation with the use of ampakines, shifting cortical neural plasticity in favor of long-term potentiation could lead to impairments in spatial memory and perhaps motor function.[22]
Cerebrolysin. Cerebrolysin, a mixture of low-molecular–weight peptides and amino acids derived from porcine brain tissue has been shown to have neuroprotective and neurotrophic properties by ameliorating sensory deficits and promoting synaptic formation and cholinergic fiber regeneration.[23] It is currently being used to treat ischemic strokes in China and Russia.1 Cerebrolysin is reported to be safe when used in combination with recombinant tissue-type plasminogen activator or cholinesterase inhibitors such as donepezil or rivastigmine.[24]
Adverse effects. Adverse reactions to cerebrolysin include vertigo, agitation, and feeling hot.
Accessibility. Nootropics are easily accessible via the internet through online vendors that appear as pharmacy websites frequently displaying images of physicians endorsing the products and promoting nootropic pharmaceutical products.[25] Nootropics, including those described above, can be ordered online without a medical prescription.[26] Nootropics are also widely available in most health and nutrition stores in many countries.
Case Reports
Case 1. A 19-year-old male college student with a history of depression and attention deficit hyperactivity disorder (ADHD) presented to the emergency department with psychosis and paranoia resulting in self-injurious behavior. His current medication was bupropion, and historically he had been prescribed methylphenidate but was no longer taking that medication. His parents reported a history of cannabis abuse but he had been abstinent for the past year. No history of psychosis was reported. Previously, the patient was functioning well, in a euthymic state, and was attending his classes. He denied any substance abuse, and urine toxicology was negative. On further questioning, the patient revealed that he was taking a supplement to treat his ADHD. He reported purchasing it online. The supplement was found to be citicoline, and he had been consuming 2 to 3 tablets three times a day for several weeks. The family had noticed some insomnia and irritability early on, but no other concerning behaviors until now. The patient was admitted to the psychiatry department, and his symptoms resolved with olanzapine. He was discharged home in a stable condition and instructed to continue taking olanzapine for one month and to stop using all supplements.
Case 2. A 24-year-old male body builder with a history of anxiety presented to the emergency room with agitation and several days of hypomania. Currently, he was not on any medications, but previously had been treated with paroxetine for anxiety. Several years ago, he used anabolic steroids for a few months, but has not used them since. He reported smoking cannabis on weekends and consuming alcohol occasionally. Urine toxicology was negative. On questioning, he reported recently using cerebrolysin to enhance his cognitive performance, consuming two tablets twice daily. The supplement was obtained from a local health store. The patient received diazepam and was observed overnight. He improved significantly and was discharged home in stable condition. Diazepam was discontinued upon discharge from the hospital. He was instructed to stop all supplements.
Case 3. A 28-year-old female graduate student presented urgently to the psychiatry clinic for new onset insomnia, anxiety, and panic attacks. She reported a history of depression that was well controlled with psychotherapy. Currently, she was not taking any medications. She denied any illicit substance use and did not consume caffeine or smoke cigarettes. She admitted to being a casual cannabis smoker, but had not been using cannabis recently. The patient reported that she recently started using armodafinil to help her cope with her stressful academic program. Initially, she consumed armodafinil on an as-needed basis, and she felt improved performance and well-being. She then started using it regularly twice daily. Approximately one week later, the symptoms of insomnia, anxiety, and panic attacks began. The patient reported using armodafinil upon the suggestion of a friend, and it was obtained online from an overseas pharmacy. The patient was advised to stop armodafinil and was prescribed clonazepam twice daily until symptoms resolved one week later. At follow-up one week later, up her symptoms had resolved.
Case 4. A 17-year-old male high school student with obsessive compulsive disorder (OCD) and learning disabilities was admitted to the emergency room and then to the psychiatry department for exacerbation of OCD with akathesia and paranoia. He had previously been maintained on fluoxetine with good control of his OCD. He reported no substance abuse or caffeine use, which was confirmed by his parents. Urine toxicology was negative. No history of psychosis or paranoia was reported by the patient or his parents. Basic medical screening showed no abnormalities. On further questioning his parents reported that he had recently started using a supplement for memory. Further investigation revealed that the supplement was piracetam. The parents had not objected to him using the supplement since they perceived it as a safe and “natural remedy.” The supplements were obtained online. His symptoms improved with daily alprazolam and olanzapine at bedtime. Fluoxetine was discontinued. He was discharged home after a short hospital stay in a stable condition on olanzapine at bedtime. Piracetam was discontinued.
Discussion
In addition to being young adults, all four of the described cases had some type of psychiatric history. Three of the four cases had a history of substance use, mainly cannabis. All of our cases reported an interest in maintaining a healthy and natural lifestyle. They did not perceive nootropics as harmful, voiced interest in “natural remedies,” and reported preferring to use supplements instead of prescription medications. They seemed not to be interested in experiencing euphoria or a pleasurable sensation, but rather to enhance their psychological or cognitive states. All cases improved rapidly and uneventfully with symptomatic treatment and discontinuation of the nootropics.
In our opinion, it is highly likely that nootropics were responsible for the psychiatric exacerbation in these cases, primarily, since they had been stable at their respective psychiatric baselines with no new psychosocial stressors or medication changes, except the initiation of nootropics. Additionally, there was no active or recent substance abuse. There is also a temporal correlation between initiating nootropics use and the psychiatric exacerbations reported.
Limitations. Two of the cases were taking psychotropic medications, which may have had drug interactions with the nootropics, causing the adverse effects. There is also the possibility of undisclosed or undetected substance abuse as a causal factor. A major limitation is the inability to definitely determine the actual composition of the nootropics, the dosing, and the frequency of use.
Conclusion
Healthcare providers in general, and specifically those in the mental health and substance abuse fields, should keep in mind that nootropic use is an under recognized and evolving problem. Nootropic use should be considered in cases where there are sudden or unexplained exacerbations of psychiatric symptoms in patients who have been stable and medication adherent. It is also important to remember that most nootropics are not detected on standard drug toxicology screening tests.
We have very little clinical information on how nootropics may interact with psychotropics (or other medications) and potentially cause adverse physical and psychiatric side effects. Finally, because nootropics are often obtained via loosely regulated sources, such as online vendors, it is possible that other psychoactive compounds are substituted for the advertised nootropics. Young adults, especially those with a history of mental health or substance use disorders, may be at particular risk of adverse effects from use of nootropics and should be educated about the potential for harm from misuse of nootropics.
References
1. Nishizaki T, Matsuoka T, Nomura T, et al. A long-term, potentiation-like facilitation of hippocampal synaptic transmission induced by the nootropic nefiracetam. Brain Res. 1999;826:281–288.
2. Turner DC, Robbins TW, Clark L, et al. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology. 2003;165(3):260–269.
3. Turner DC, Clark L, Dowson J, et al. Modafinil improves cognition and response inhibition in adult attention- deficit/hyperactivity disorder. Biol Psychiatry. 2004;55:1031–1040.
4. Ferraro L, AntonelliT, Tanganelli S, et al. The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABAA receptor blockade. Neuropsychopharmacology. 1999;20:346–356.
5. Darwish M, Kirby M, Hellriegel ET, Robertson P Jr. Armodafinil and modafinil have substantially different pharmacokinetic profiles despite having the same terminal half-lives. Clin Drug Investig. 2009;29(9):613–623
6. Greve DN, Duntley SP, Larson-Prior L, et al. Effect of armodafinil on cortical activity andworking memory in patients with residual excessive sleepiness associated with CPAP-Treated OSA: a multicenter fMRI study. J Clin Sleep Med. 2014;10(2):143–153.
7. Wignall ND, Brown ES. Citicoline in addictive disorders: a review of the literature. Am J Drug Alcohol Abuse. 2014;40(4):262–268.
8. Spiers PA, Hochanadel G. Citicoline for traumatic brain injury: report of two cases, including my own. J Int Neuropsychol Soc. 1999;5:260–264
9. Cho HJ, Kim YJ. Efficacy and safety of oral citicoline in acute ischemic stroke: drug surveillance study in 4,191 cases. Methods Find Exp Clin Pharmacol. 2009;31:171–176.
10. Yoon SJ1, Lyoo IK, Kim HJ, et al. Neurochemical alterations in methamphetamine-dependent patients treated with cytidine-5’- diphosphate choline: a longitudinal proton magnetic resonance spectroscopy study. Neuropsychopharmacology. 2009;35(5)1165–1173.
11. Waegemans T,Wilsher CR, Danniau A, Ferris SH, et al. Clinical efficacy of piracetam in cognitive impairment: a meta-analysis. Dement Geriatr Cogn Disord. 2002;13:217–224
12. Ahmed AH, Oswald RE. Piracetam defines a new binding site for allosteric modulators of alphaamino-3- hydroxy-5-methyl-4- isoxazole-propionic acid (AMPA) receptors. J Med Chem. 2010;53(5):2197–2203.
13. Cohen SA, Müller WE. Effects of piracetam on Nmethyl-D-aspartate receptor properties in the aged mouse brain. Pharmacology. 1993;47:217–222.
14. Corazza O, Assi S, Simonato P, et al. Promoting innovation and excellence to face the rapid diffusion of novel psychoactive substances (NPS) in the EU: the outcomes of the ReDNet project. Hum Psychopharmacol. 2013;28(4):317–323.
15. Brandao F, Cadete-Leite A, Andrade JP, et al. Piracetam promotes mossy fiber synaptic reorganization in rats withdrawn from alcohol. Alcohol. 1996;13:239–249.
16. Brandao F, Paula-Barbosa MM, Cadete-Leite A. Piracetam impedes hippocampal neuronal loss during withdrawal after chronic alcohol intake. Alcohol. 1995;12:279–288.
17. Drugs-forum. Another pirpacetam experiment. 2006. http://www.drugs-forum.com/ forum/showthread.php?t=136253. Accessed June 25, 2013.
18. Drugs-forum. LSD piracetam combo. 2005. http://www.drugsforum. com/forum/showthread. php?t=6977. Accessed July 11, 2013.
19. Drugs-forum. High-dose piracetam: my findings. 2011. http://www.drugsforum. com/forum/show thread.php?t=153468. Accessed June 6, 2013
20. Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets 2007;8:583–602.
21. Wezenberg E, Verkes RJ, Ruigt GS, et al. Acute effects of the ampakine farampator on memory and information processing in healthy elderly volunteers. Neuropsychopharmacology. 2007;32:1272–1283.
22. Aiba A, Kano M, Chen C, et al. Deficient cerebellar long-term depression and impaired motor learning in mGluR1mutantmice. Cell 1994;79:377–388.
23. Satou T, Itoh T, Tamahai Y, et al. Neurotrophic effects of FPF 1070 (Cerebrolysin®) on cultured neurons from chicken embryo dorsal root ganglia, ciliary ganglia, and sympathetic trunks. J Neural Transm. 2000;107:1253–1262.
24. Thome J, Doppler E. Safety profile of Cerebrolysin: clinical experience from dementia and stroke trials. Drugs Today (Barc). 2012;48(Suppl A):63–69.
25. Mechaeil R, Gard P, Jackson A, Rusted J. Cognitive enhancement following acute losartan in normotensive young adults. Psychopharmacology. 2011;217(1):51–60.
26. Orizio G, Schulz P, Domenighini S, et al. Cyberdrugs: a cross-sectional study of online pharmacies characteristics. Eur J Publ Health. 2009;19(4):375–377.





