 by Randy A. Sansone, MD, and Lori A. Sansone, MD R. Sansone is a professor in the Departments of Psychiatry and Internal Medicine at Wright State University School of Medicine in Dayton, OH, and Director of Psychiatry Education at Kettering Medical Center in Kettering, OH. L. Sansone is a civilian family medicine physician and Medical Director of the Family Health Clinic at Wright-Patterson Air Force Base Medical Center in WPAFB, OH. The views and opinions expressed in this article are those of the authors and do not reflect the official policy or position of the United States Air Force, Department of Defense, or United States Government. Innov Clin Neurosci. 2014;11(3–4):37–42
by Randy A. Sansone, MD, and Lori A. Sansone, MD R. Sansone is a professor in the Departments of Psychiatry and Internal Medicine at Wright State University School of Medicine in Dayton, OH, and Director of Psychiatry Education at Kettering Medical Center in Kettering, OH. L. Sansone is a civilian family medicine physician and Medical Director of the Family Health Clinic at Wright-Patterson Air Force Base Medical Center in WPAFB, OH. The views and opinions expressed in this article are those of the authors and do not reflect the official policy or position of the United States Air Force, Department of Defense, or United States Government. Innov Clin Neurosci. 2014;11(3–4):37–42
This ongoing column is dedicated to the challenging clinical interface between psychiatry and primary care—two fields that are inexorably linked.
Funding: There was no funding for the development and writing of this article.
Financial disclosures: The authors have no conflicts of interest relevant to the content of this article.
Key words: Antidepressants, desvenlafaxine, duloxetine, levomilnacipran, milnacipran, selective serotonin reuptake inhibitors, SNRIs, venlafaxine
Abstract: The serotonin norepinephrine reuptake inhibitors are a family of antidepressants that inhibit the reuptake of both serotonin and norepinephrine. While these drugs are traditionally considered a group of inter-related antidepressants based upon reuptake inhibition, they generally display different chemical structures as well as different pharmacological properties. In this article, we discuss these and other differences among the serotonin norepinephrine reuptake inhibitors, including the year of approval by the United States Food and Drug Administration, generic availability, approved clinical indications, half-lives, metabolism and excretion, presence or not of active metabolites, dosing schedules, proportionate effects on serotonin and norepinephrine, and the timing of serotonin and norepinephrine reuptake (i.e., sequential or simultaneous). Again, while serotonin norepinephrine reuptake inhibitors are grouped as a family of antidepressants, they exhibit a surprising number of differences—differences that may ultimately relate to clinical nuances in patient care.
Introduction
In this edition of The Interface, we compare and contrast several characteristics and pharmacological properties of the serotonin norepinephrine reuptake inhibitors (SNRIs), which include venlafaxine immediate release (IR) and extended release (XR), duloxetine, desvenlafaxine, milnacipran, and levomilnacipran. Each of these antidepressants inhibits the reuptake of both serotonin and norepinephrine. However, in addition to their generally unique chemical structures, they exhibit a number of distinguishing pharmacological characteristics, as well. We will highlight these differences and identify potential clinical implications when relevant.
Venlafaxine
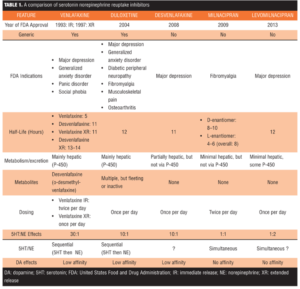
Venlafaxine immediate release (Effexor™) was the first SNRI to be marketed in the United States and was initially approved by the United States Food and Drug Administration (FDA) in 1993. The debut of venlafaxine IR, which is dosed twice per day, was followed in 1997 with the launch of a micro-encapsulated XR formulation (Effexor XR™), which is dosed once per day.[1] The clinical advantage of the XR formulation is less nausea and dizziness at the outset of therapy,[2] but the capsule cannot be breached prior to or during ingestion. Venlafaxine is a bicyclic (2 chemical rings) and is structurally different than the other SNRIs. Venlafaxine has four approved clinical indications through the FDA: major depression, generalized anxiety disorder, panic disorder, and social phobia.[1] Venlafaxine IR and venlafaxine XR are both available as brand and generic formulations.
Pharmacokinetics. The half-life of the immediate release formulation of venlafaxine is five hours. The active metabolite of venlafaxine, o-desmethylvenlafaxine or desvenlafaxine, has a half-life of 11 hours. The XR formulation of venlafaxine demonstrates somewhat longer half-lives for both venlafaxine and desvenlafaxine at 11 hours and 13 to 14 hours, respectively.[2] Venlafaxine is primarily metabolized through the liver (2D6, 3A3/4 isoenzymes),[2] and is therefore liable to cause drug interactions1 as well as be metabolically susceptible to genetic polymorphism (2D6).
Reuptake effects. Venlafaxine inhibits the reuptake of both serotonin and norepinephrine in a disproportionate manner. Explicitly, venlafaxine has a 30-fold higher affinity for the reuptake inhibition of serotonin compared to norepinephrine.[3] In addition, venlafaxine inhibits serotonin and norepinephrine reuptake in a sequential manner, such that serotonin reuptake is initially inhibited, followed by norepinephrine reuptake inhibition.[4] This finding supports the general clinical experience with venlafaxine—that initial side effects are predominantly related to serotonin (e.g., headaches, nausea, fatigue, sexual dysfunction) whereas subsequent side effects with higher dosing are related to both serotonin and norepinephrine (e.g., activation effects, dry mouth, night sweats). At high doses, venlafaxine may exhibit some dopamine reuptake inhibition.[1]
Duloxetine
Duloxetine (Cymbalta™) was the second SNRI to be approved by the FDA for use in the United States (2004).[5] One early clinical indication for duloxetine was diabetic peripheral neuropathy—the first drug in the United States to be approved for this condition.[5] Since its introduction, duloxetine has also received approval by the FDA for major depression, generalized anxiety disorder, fibromyalgia, musculoskeletal pain, and osteoarthritis.[5] As a result, duloxetine has the most FDA-approved indications of any SNRI. Unlike venlafaxine, duloxetine has garnered a number of clinical indications for nonpsychiatric conditions—each representing a different type of pain syndrome. Duloxetine, which is structurally different than venlafaxine, has three rings in its chemical structure, two of which are adjacent to each other.[5] Duloxetine became a candidate for generic formulation in late 2013.
Pharmacokinetics. The half-life of duloxetine is around 12 hours. Although the metabolism of duloxetine results in a number of metabolites, these are either ephemeral or lack meaningful biological activity—i.e., the metabolites of duloxetine have little-to-no genuine clinical activity.[6,7] Duloxetine is metabolized mainly through the hepatic P-450 isoenzyme system (2D6, 1A2 isoenzymes), which indicates a potential risk for drug interactions as well as a metabolic susceptibility to genetic polymorphism (2D6).[5] Dosing is once per day.
Reuptake effects. Like venlafaxine, which demonstrates a dominant serotonergic influence in comparison to its noradrenergic influence, duloxetine maintains this serotonergic predominance but to a lesser degree. Specifically, duloxetine demonstrates a 10-fold higher selectivity for serotonin reuptake inhibition compared with norepinephrine reuptake inhibition.[8] Similar to venlafaxine, duloxetine’s invocation of reuptake inhibition is asymmetrical, with an initial influence on serotonin followed by a subsequent influence on norepinephrine.[9] Duloxetine demonstrates a low but apparent affinity for dopamine.[5]
Desvenlafaxine
Desvenlafaxine (Pristique™) was the third SNRI to receive approval from the FDA for use in the United States, which occurred in 2008.[10] Desvenlafaxine is the single active metabolite of venlafaxine and therefore demonstrates some structural similarities with venlafaxine, including two chemical rings that are not adjacent to each other. Desvenlafaxine is solely manufactured as a sustained-release tablet and is clinically indicated by the FDA for the treatment of major depression.[10] Desvenlafaxine is not available in a generic form and the patent will not expire for several years.
Pharmacokinetics. The elimination half-life of desvenlafaxine is 11 hours.10 Desvenlafaxine is partially metabolized through conjugation and partially metabolized through the P-450 isoenzyme system (3A4); however, nearly 50 percent of the drug is excreted unchanged in the urine.[11,12] The high rate of excretion of unchanged drug in the urine indicates that desvenlafaxine undergoes marginal metabolism through the P-450 isoenzyme system;[11,13] likewise, the conjugated metabolite is not pharmacologically active.[14] Because of this pharmacokinetic profile (i.e., little interface with the P-450 isoenzyme system), desvenlafaxine would be expected to have a reduced risk for potential drug interactions in comparison with the preceding SNRIs.[15] Also, desvenlafaxine is not subject to 2D6 influences (i.e., genetic polymorphism). The dosing of desvenlafaxine is once per day.
Reuptake effects. According to in-vitro studies, desvenlafaxine demonstrates a 10-fold higher selectivity for serotonin reuptake inhibition compared with norepinephrine reuptake inhibition,[16,17] which indicates a profile similar to duloxetine. With regard to the timing of desvenlafaxine’s effect on serotonin and norepinephrine reuptake inhibition—either sequential like venlafaxine and duloxetine or simultaneous like milnacipran (see below)—we were unable to locate any empirical data in this regard. Like venlafaxine and duloxetine, desvenlafaxine demonstrates a weak inhibitory effect on the reuptake of dopamine.[1]
Milnacipran
Milnacipran (Savella™) was the fourth SNRI to be introduced in the United States (2009).[18] At the present time, milnacipran is only indicated by the FDA for the treatment of fibromyalgia. However, milnacipran has been available in France since 1997 for the treatment of major depression.[19] Milnacipran is not available in a generic formulation and the expiration of the patent is not imminent.
Pharmacokinetics. Milnacipran is a racemic mixture composed of d-milnacipran and l-milnacipran.[18] D-milnacipran has a half-life of 8 to 10 hours whereas l-milnacipran has a half-life of 4 to 6 hours.[24] Unlike the other SNRIs, which entail once-daily dosing, the dosing of milnacipran is twice-per-day. Milnacipran undergoes conjugation in the liver and it is devoid of significant interactions with the P-450 isoenzymes, suggesting few if any drug interactions[17] and no susceptibility to 2D6 genetic polymorphism. Milnacipran has no significant active metabolites.[17] Most of the administered drug is excreted in the urine,[17] either as the parent compound (55%) or as several inactive metabolites.[18]
Reuptake effects. Milnacipran is the most balanced reuptake inhibitor among the current SNRIs, with nearly equipotent reuptake inhibition of serotonin and norepinephrine.[19] According to some sources, milnacipran may even have slightly more noradrenergic effects than serotonergic effects—up to three-fold higher.[8,18] Unlike the previously discussed SNRIs, milnacipran has no effects on dopamine.[8,18] In addition, rather than the sequential effects on serotonin and norepinephrine that are observed with venlafaxine and duloxetine, milnacipran exerts an equivalent or simultaneous effect on reuptake inhibition of both neurotransmitters at all doses.[17]
Levomilnacipran
Levomilnacipran (Fetzima™) is the most recently available product in the SNRI line. Levomilnacipran was approved by the FDA in 2013 for the treatment of major depression. It is the more active l-enantiomer of milnacipran. Levomilnacipran has been developed solely as a sustained-release formulation (once per day).[20,21] This pharmacological maneuver is likely to improve patient adherence in comparison with milnacipran, which is dosed twice per day. In addition, the sustained release capsule enables better marketability as the remaining SNRIs are dosed once per day. The patent expiration is presently projected at 2023.
Pharmacokinetics. The half-life of levomilnacipran is approximately 12 hours.[22] Levomilnacipran undergoes desethylation, which is primarily through the 3A4 isoenzyme, as well as hydroxylation.[22] The resulting metabolites are inactive.[22] Nearly 60 percent of levomilnacipran is excreted unchanged in the urine.[22]
Reuptake effects. Unique among the SNRIs, levomilnacipran demonstrates a two-fold greater potency for norepinephrine reuptake inhibition in comparison to serotonin reuptake inhibition.[20] Like milnacipran, there are no effects on dopamine and levomilnacipran is likely to exert an equivalent or simultaneous reuptake inhibition effect on both serotonin and norepinephrine at all doses.
Clinical Comparisons
Venlafaxine was the first SNRI to debut in the United States, followed by duloxetine, desvenlafaxine, milnacipran, and levomilnacipran. Of the available SNRIs, both venlafaxine and duloxetine are available in generic formulations, suggesting a potential cost advantage. Among the SNRIs, duloxetine has the most clinical indications through the FDA (6 indications), followed by venlafaxine (4 indications), and desvenlafaxine, milnacipran, and levomilnacipran (one indication each). In comparison with other types of antidepressants, such as the selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants, the SNRIs have relatively short half-lives, varying from 8 to 14 hours, and few-to-no active metabolites, suggesting a simpler pharmacology. In terms of metabolism, both venlafaxine and duloxetine are metabolized through the P-450 isoenzyme system, indicating the possibility of potential drug interactions as well as metabolic susceptibility to 2D6 genetic polymorphism. In contrast, desvenlafaxine, milnacipran, and levomilnacipran largely bypass the P-450 isoenzyme system and undergo conjugation; therefore, they are less likely to precipitate drug interactions. Of the five SNRIs, only venlafaxine has an active metabolite (desvenlafaxine)—a further testament to the simpler pharmacology of the SNRIs. Among the five SNRIs, only milnacipran and venlafaxine IR are dosed twice per day whereas the remaining four, including venlafaxine XR, are dosed once per day. While all SNRIs are serotonin/norepinephrine reuptake inhibitors, each has a different proportionate effect or influence on reuptake inhibition. Explicitly, venlafaxine exerts more potent effects on serotonin reuptake inhibition than norepinephrine reuptake inhibition. Both duloxetine and desvenlafaxine demonstrate less imbalance, but still retain greater serotonin than norepinephrine reuptake inhibition. In contrast, milnacipran exerts a relatively equal influence on serotonin and norepinephrine reuptake inhibition whereas levomilnacipran demonstrates a reversed profile, with greater norepinephrine reuptake inhibition than serotonin reuptake inhibition. Whether these disproportionate influences among the SNRIs will yield any meaningful clinical differences is yet to be determined. However, such differences indicate that these drugs are clearly dissimilar from one another. Finally, both venlafaxine and duloxetine exhibit dose-related sequential effects on reuptake inhibition, first affecting serotonin and then norepinephrine; this results in a sequential side-effect profile, as well, with the initial onset of serotonergic side effects followed by noradrenergic side effects. While the status of desvenlafaxine in this regard is unclear, milnacipran and likely levomilnacipran act simultaneously on serotonin and norepinephrine reuptake inhibition. Overall, the SNRIs have minimal influences on dopamine, or other types of receptors for that matter.
Conclusion
While the SNRIs are traditionally viewed as a distinct family of antidepressants, these drugs harbor a number of pharmacological differences, fostering a unique identity for each within the group. Whether these differences will translate into meaningful clinical differences remains unknown. However, a working knowledge of the pharmacological differences among the SNRIs provides the necessary groundwork for evaluating the clinical relevance and niche for each—a process that is germane for both primary care and psychiatric clinicians.
References
1. Gold Standard, Inc. Venlafaxine. Clinical Pharmacology [database online]. http://www.clinicalpharmacology.com. Accessed on 7/9/13. 2. Olver JS, Burrows GD, Norman TR. The treatment of depression with different formulations of venlafaxine: a comparative analysis. Hum Psychopharmacol. 2004;19:9–16.
3. Montgomery SA. Tolerability of serotonin norepinephrine reuptake inhibitor antidepressants. CNS Spectr. 2008;13:27–33.
4. Janicak PG, Davis JM, Preskorn SH, Ayd FJ Jr. Principles and Practice of Psychopharmacotherapy. 2nd ed. Baltimore: Lippincott, Williams & Wilkins; 1997.
5. Gold Standard, Inc. Duloxetine. Clinical Pharmacology [database online].http://www.clinicalpharmacology.com. Accessed on 7/9/13.
6. Kuo F, Gillespie TA, Kulanthaivel P, et al. Synthesis and biological activity of some known and putative duloxetine metabolites. Bioorg Med Chem Lett. 2004;14:3481–3486.
7. Hunziker ME, Suehs BT, Bettinger TL, Crismon ML. Duloxetine hydrochloride: a new dual-acting medication for the treatment of major depressive disorder. Clin Ther. 2005;27:1126–1143.
8. Stahl SM, Grady MM, Moret C, Briley M. SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005;10:732–747.
9. Turcotte JE, Debonnel G, de Montigny C, et al. Assessment of the serotonin and norepinephrine reuptake blocking properties of duloxetine in healthy subjects. Neuropsychopharmacology. 2001;24:511–521.
10. Gold Standard, Inc. Desvenlafaxine. Clinical Pharmacology [database online].http://www.clinicalpharmacology.com. Accessed on 7/9/13.
11. Perry R, Cassagnol M. Desvenlafaxine: a new serotonin-norepinephrine reuptake inhibitor for the treatment of adults with major depressive disorder. Clin Ther. 2009;31:1374–1404.
12. Desvenlafaxine Package Insert. http://labeling.pfizer.com/showlabeling.aspx?id=497. Accessed on 7/15/13.
13. Lourenco MT, Kennedy SH. Desvenlafaxine in the treatment of major depressive disorder. Neuropsychiatr Dis Treat. 2009;5:127–136.
14. Cohen LJ. Desvenlafaxine: frequently asked questions. Prim Psychiatry. 2009;16:1–8.
15. Seo HJ, Sohi MS, Patkar AA, et al. Desvenlafaxine succinate: a newer antidepressant for the treatment of depression and somatic symptoms. Postgrad Med. 2010;122:125–138.
16. Deecher DC, Beyer CE, Johnston G, et al. Desvenlafaxine succinate: a new serotonin and norepinephrine reuptake inhibitor. J Pharmacol Exp Ther. 2006;318:657–665.
17. Kasper S, Pail G. Milnacipran: a unique antidepressant? Neuropsychiatr Dis Treat. 2010;6:S23-31.
18. Gold Standard, Inc. Milnacipran. Clinical Pharmacology [database online].http://www.clinicalpharmacology.com. Accessed on 7/9/13.
19. Delini-Stula A. Milnacipran: an antidepressant with dual selectivity of action on noradrenaline and serotonin reuptake. Hum Psychopharmacol. 2000;15:255–260.
20. Auclair AL, Martel JC, Assie MB, et al. Levomilnacipran (F2695), a norepinephrine-preferring SNRI: profile in vitro and in models of depression and anxiety. Neuropharmacology. 2013;70:338–347.
21. Clinical Trials.Gov. Safety and efficacy of levomilnacipran SR in major depressive disorder. http://clinicaltrials.gov/ct2/show/NCT01377194. Accessed on 7/17/13.
22. Forest Pharmaceuticals, Inc. Highlights of prescribing information. https://www.fetzima.com/?guid=google577. Accessed on 7/31/13.