by David V. Sheehan, MD, MBA; Jennifer M. Giddens; and Ivan Sascha Sheehan, PhD
by David V. Sheehan, MD, MBA; Jennifer M. Giddens; and Ivan Sascha Sheehan, PhD
Dr. D. Sheehan is Distinguished University Health Professor Emeritus at the University of South Florida College of Medicine, Tampa, Florida; J. Giddens is the Co-founder of the Tampa Center for Research on Suicidality, Tampa, Florida; and Dr. I. S. Sheehan is Assistant Professor and Graduate Program Director, School of Public and International Affairs, University of Baltimore, Baltimore, Maryland.
Innov Clin Neurosci. 2014;11(9–10):93–140
Funding: There was no funding for the development and writing of this article.
Financial Disclosures: Dr. D. Sheehan is the author and copyright holder of the Sheehan-Suicidality Tracking Scale (S-STS), the Sheehan-Suicidality Tracking Scale Clinically Meaningful Change Measure Version (S-STS CMCM), the Pediatric versions of the S-STS, the Sheehan Disability Scale (SDS), and the Suicidality Modifiers Scale; is a co-author of the Suicide Plan Tracking Scale (SPTS), Sheehan-Homicidality Tracking Scale, and the Mini International Neuropsychiatric Interview (MINI); and owns stock in Medical Outcomes Systems, which has computerized the S-STS and the MINI. J. Giddens is the author and copyright holder of the SPTS and is a named consultant on the S-STS, the S-STS CMCM, the Pediatric versions of the S-STS, and the Suicidality Modifiers Scale. Dr. I. S. Sheehan is the co-author of the Sheehan-Homicidality Tracking Scale. He is also the son of Dr. D. Sheehan, who is the author and copyright holder of the S-STS, the S-STS CMCM, the Pediatric versions of the S-STS, the SDS, and the Suicidality Modifiers Scale; a co-author of the SPTS, the Sheehan-Homicidality Tracking Scale, and the MINI; and owns stock in Medical Outcomes Systems, which has computerized the S-STS and the MINI.
Key Words: Suicide scale, suicide assessment, suicide risk, suicide, suicidality, suicidality scale, patient-rated suicide assessment, pediatric suicide, suicide in children, pediatric suicide scale, adolescent suicide scale, S-STS, homicide assessment, homicidality, homicide, S-HTS
Abstract: There is a need for a choice of scales to evaluate the full range of suicidal phenomena. Such scales must be capable of use as both safety and efficacy outcome measures in research and in clinical settings. Central to the success in finding and developing effective anti-suicidal medications is having a sensitive suicidality scale that can detect an efficacy signal in conventional sample sizes used in clinical trials. The Sheehan-Suicidality Tracking Scale was developed for these purposes. This article provides a 2014 status update on the scale’s progress, its use, and its properties. The authors review why and how the scale was developed; the scale structure, versions, and properties; the trials in which it was used; the time frames accommodated; its validation and reliability studies; its utility in screening and assessment; its utility in assessing treatment-emergent suicidal adverse events; its use as an efficacy outcome measure; its availability in self-rated and clinician-rated forms; the availability and linguistic validation of pediatric versions; linguistic validation in other languages; how it compares with global ratings of suicidality; and its possible utility and applications.
Introduction
There is a need for a choice of scales to evaluate the full range of suicidal phenomena. Such scales must be capable of use as both safety and efficacy outcome measures in research and in clinical settings. Sensitivity to anti-suicidal effects in modest sample sizes is particularly important in the context of efforts to find and develop anti-suicidal medications. The Sheehan-Suicidality Tracking Scale (S-STS) was developed to provide a brief but efficient assessment instrument for use in assessing change in suicidal ideation and behavior while providing a comprehensive description of suicidal ideation and behavior. The primary goals in the design of the S-STS were for the scale to be as follows:
1. Short and inexpensive
2. Simple, clear, and easy to administer or self-rate
3. Highly sensitive (i.e., able to detect a high proportion of patients who are suicidal)
4. Specific (i.e., able to screen out those who are not suicidal)
5. Sensitive to change in suicidal ideation and behavior
6. Compatible with the United States Food and Drug Administration (FDA) categories for prospective assessment of suicidal ideation and behavior[1,2]
7. Useful in clinical as well as research settings
8. Useful in detecting an efficacy signal for anti-suicidal medications
9. Capable of use in pediatric and geriatric settings.
Because of the risks associated with suicidality and in the interest of safety, the expectations and hurdles for a suicide assessment scale are higher than for other scales in psychiatry. To meet these expectations and in response to much valuable feedback, the S-STS has evolved over time. This article aims to provide a 2014 status update on the scale, its properties, and its potential uses.
Why and How was the S-STS Developed?
Suicide is a leading cause of premature death among psychiatric patients and a leading source of malpractice suits against psychiatrists and mental health professionals.[3–5] A suicidality module was included in the Mini International Neuropsychiatric Interview (MINI)[6] (a structured diagnostic interview coauthored by author D.S.) as early as 1992, because other structured diagnostic interviews did not provide this. The suicidality module, like the other modules in the MINI, is in a yes/no response format. Subsequently, the first author of this article (D.S.), who developed the scales in the MINI Tracking Scales, changed the MINI modules, including the suicidality module, into a set of Likert scales for treatment-outcome tracking. With the FDA expectations of more thorough suicide assessments in clinical trials, several sponsors asked the first author of this article (D.S.) to accommodate all the FDA suicide assessment expectations into the suicidality module of the MINI Tracking. In this process, the S-STS was developed as a separate scale. As it was modified, we modified the MINI suicidality module to keep pace with changes in the S-STS. The MINI 7 structured diagnostic interview for the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) is now fully compatible item by item with the S-STS. The S-STS scale evolved further with considerable and thoughtful feedback from regulatory agencies, patients, sponsors, and researchers to its current state. The scale author’s consistent primary goal was to develop a scale that could sensitively discriminate anti-suicidal properties between drug and placebo in standard clinical trial sample sizes. In addition, the scale should serve as a sensitive, adverse-event, suicidality detector in modest samples.
Current Scale Variants
Standard S-STS. The standard version of the S-STS (Appendix A [CLICK TO ACCESS]) is a 16-item scale that assesses the seriousness of suicidality phenomena on a Likert-type scale (0–4) ranging from “not at all” (0) to “extremely” (4). It also assesses the frequency of key phenomena and the overall time spent in suicidality. The standard version is available in clinician- and patient-rated formats. These formats are identical, except that the clinician rates the former and the patient rates the latter. There is also a “reconciled” version, which can be used to reconcile discrepancies, if they exist, between the patient and clinician on specific items. The standard S-STS can be used for screening and other purposes (e.g., tracking suicidality as an adverse effect in trials or in clinical practice). Page 1 of the two-and-a-half pages layout can be used as a stand-alone suicidality tracking scale for clinical practice settings. The last page (page 3) is only completed if the patient misses a follow-up appointment and is unavailable to allow completion of the scale. This section captures the best available information on the patient’s disposition. This information is usually required by regulatory agencies to close the loop as completely as possible on the outcome in a clinical trial in relation to suicidality.
The Clinically Meaningful Change Measure (CMCM) version of the S-STS (Appendix B [CLICK TO ACCESS]). This version is a much more expanded version developed for specific testing of the anti-suicidal effects of medications. The main reason for developing this variant was to address an expectation of European regulators that any drug seeking regulatory approval for an anti-suicidal indication needs to demonstrate a clinically meaningful change in addition to showing statistically significant superiority over a placebo on a suicide ideation and behavior scale, given the risks and gravity involved in suicidality. Assessment of clinically meaningful change has become increasingly important for treatment planning, monitoring progress, and evaluating treatment response in clinical trials and in clinical practice. Minimally important change has been defined as “the difference in score on a health-related […] instrument that corresponds to the smallest change in status that stakeholders (persons, patients, significant others, or clinicians) consider important.”[7] Clinically meaningful or important change can facilitate interpretation of scores obtained on self-report measures of health status used to assess treatment effects at the aggregate and individual level.[8]
An anti-suicidal medication might show a statistically significant difference compared to placebo, but that change might not also be clinically meaningful. The effects of such an anti-suicidal medication should be impressive enough so that it is able to alter the clinician’s judgment of risk and decisions about the acute clinical management or disposition of the case. The S-STS CMCM is designed to meet that need. Appendix B [CLICK TO ACCESS] shows the additional domains that should be altered by an anti-suicidal treatment and how these domains are measured, anchored, and statistically analyzed (as seen in Appendix C [CLICK TO ACCESS]) in a way that any clinician could judge the extent of an anti-suicidal medication’s clinically meaningful effect.
The CMCM version of the S-STS has four parts. The first section can be either patient-rated or clinician-rated. This section is identical to the first two pages of the standard S-STS.
The second section is patient-rated. It provides patients with a series of additional ratings. These include 1) an opportunity to rate a series of risk or protective factors that might be important aggravating or relieving factors in the subject’s suicidal ideation and behaviors; 2) a series of 11-point (0–10) discretized visual analog (DISCAN) scales on which patients can rate their ability and willingness to cope with their suicidality, their ability and willingness to “stay safe,” the extent to which their suicidality is deliberate, the extent to which it is impulsive, the extent to which it has impacted the quality of their lives, and the extent to which it has impaired their work, social, or family lives; and 3) a patient-rated global severity of suicidal impulses, thoughts, and behaviors rating and an opportunity to provide a self-assessment of treatment needs.
The third section of the CMCM version is clinician-rated. This section gives the clinician an opportunity to rate his or her judgment of the patient’s suicide risk and a judgment of level of management required for the patient’s suicidal ideation and behavior. It also captures a global assessment of suicidality based on all the information collected in the earlier sections of the scale with additional input from others and from any additional probe questions the clinician deems necessary to complete the assessment.
The fourth section is completed by the clinician only if the patient misses a follow up appointment and is unavailable, which allows completion of the scale. It is identical to page 3 of the S-STS standard version.
The Pediatric versions of the S-STS. The Pediatric versions of the S-STS were developed specifically for children and adolescents. There are currently three pediatric forms of the S-STS. One is for 6- to 8-year-olds, one for 9- to 12-year-olds, and one for 13- to 17-year-olds. A novel method of linguistic validation was used in the development of these versions. To our knowledge, this makes these three pediatric S-STS versions the first linguistically validated versions of a suicidality scale in children and adolescents. The Pediatric versions of the S-STS are the subject of a separate article.[9]
Scale Properties
Response format. To capture the seriousness and intensity of each suicidality phenomenon, the S-STS uses a Likert-like response format ranging from “not at all” (0), “a little” (1), “moderately” (2), “very” (3), to “extremely” (4). This response format allows the scale to capture finer discriminations for tracking than the dichotomous yes/no format used in the suicidality module of the MINI and in the scale’s most widely used alternative, the Columbia–Suicide Severity Rating Scale (C-SSRS).[10] The C–SSRS, in contrast, captures an intensity rating on the highest-rated single combination of a limited number of six of 32 possible suicidal ideation combinations. The S-STS captures the seriousness of all suicidal phenomena.
Scale structure. The C–SSRS has features of a Guttman scale structure, where the suicidal ideation items are ordered so that an individual who agrees to a particular item (e.g., suicidal method, intent, or plan) is assumed to have agreed with a lower ordered item (e.g., active suicidal ideation). In contrast, the S-STS scale structure treats each item separately without any assumptions of inherent order or cumulativeness. The S-STS structure makes it possible for the scale to capture a wider range of combinations of suicidal ideation and behavior than the C–SSRS and to assess the seriousness of each ideation item separately.
Time frames. The S-STS accommodates a wide range of time frames. In clinical trials, the frequently used variants are “in the past week,” “in the past month,” “since the last visit,” “lifetime look back,” and “in the past day.” In emergency room settings, the scale can be adapted to cover all phenomena relating to “your most recent attempt” or to “what brought you here.” A shorter adaptation can be used to assess suicidality in the past 15 minutes, in the past hour, or for other shorter times frames that might be needed for testing efficacy of medications with rapid anti-suicidal properties. These are all outlined in the scoring instructions (Appendix C [CLICK TO ACCESS]) and in the instructions for use in acute rapid onset of action studies (Appendix D [CLICK TO ACCESS]).
Administration time. In a clinical sample of 34 patients with reported suicidal ideation or behavior, the average time taken to administer the standard S-STS was 9.1 minutes.[11] While this duration was slightly longer than that reported for the C-SSRS (8.1 minutes) in the same study, it was comparable to the duration reported for suicidal subjects in another study of the C–SSRS (9.6 minutes)[12] and somewhat shorter than that for the InterSePT Scale for Suicidal Thinking-Plus (ISST-Plus) (14.5 minutes).[11] The patient-rated version of the S-STS took 8.3 minutes and the reconciliation version took 2.5 minutes in the same study. In a sample of generalized anxiety disorder patients in a clinical trial who did not have active suicidal ideation at study entry, the original eight-item version of the S-STS took 1 to 2 minutes to complete.[13]
Screening, study exclusion, and monitoring alert rules. As shown in Appendix C [CLICK TO ACCESS], the standard S-STS can be used to determine if a patient should be excluded from a study at the screening visit or alternatively after the study start and/or if a monitor should be alerted. Although different protocols have different needs, we provide suggestions based on item scores for these contingencies. Appendix E [CLICK TO ACCESS] provides suggested guidelines on study stopping rules to be used with the S-STS.
Scoring. As shown in Appendix C [CLICK TO ACCESS], the standard S-STS can be used to generate a summated score (total score), individual factor scores for suicidal ideation, suicidal intent, suicidal planning, suicidal behavior, and non-suicidal self-injury. Counts for suicidal ideation events; preparatory acts; suicide attempt events; non-suicidal self-injury events; and usual, most, and least amount of time in suicidal ideation, impulses, or behavior can also be calculated from the standard S-STS. The CMCM version generates all of the same scores and counts. In addition, it can be used to generate total risk and total protective factor scores, a total clinically meaningful impairment score, a functional impairment score, and a global severity score.
Mapping to FDA algorithms. The S-STS accommodates the definitions used in the Columbia Classification Algorithm of Suicide Assessment (C-CASA)[14] and adopted by the FDA in its 2010 Draft Guidance for assessment of suicidal ideation in clinical trials.1 It also maps to the expanded classification algorithm for suicide assessment adopted by the FDA in its updated Draft Guidance issued in 2012 (referred to as FDA-CASA 2012).[2] (The mapping tables of the S-STS to the FDA 2010 and 2012 Draft Guidance categories are in Appendices F and G [CLICK TO ACCESS].)
Linguistic validation in other languages. The S-STS has been linguistically validated in over 20 languages by Mapi Group (www.mapigroup.com). This is expected to increase to up to 70 languages in the near future.
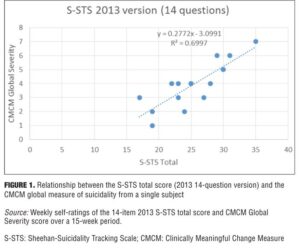
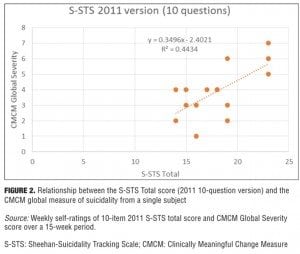
Changes in S-STS items from 2011 to 2013. The 2009 version of S-STS had 10 items. In 2011, the method item was disaggregated to improve sensitivity into two items: one for method (how) and one for means (with what). In 2012, we realized that this version was missing something that was captured in global suicide severity ratings and that its sensitivity could still be improved. By disaggregating one additional planning item into two questions—one for the location and another for the timing of plan—we were able to improve the relationship between the S-STS total score and the global severity of suicidality, making it more sensitive as a research tool in detecting efficacy and safety signals. As shown in Figure 1, there was a direct ascending linear relationship between S-STS total score and the global suicidality severity rating (R2=0.6997) for the 14-item 2013 version. The relationship was less clear (R2=0.4434) for the 10-item 2011 version (Figure 2) over exactly the same concurrent timeframe.
Validity and Reliability Testing
Evidence of validity. Coric et al[13] incorporated the self-rated S-STS into a multicenter, randomized, double-blind, placebo-controlled, and active comparator eight-week trial examining BMS 562086, escitalopram, and placebo for general anxiety disorder (GAD). Eighty-two subjects completed 297 S-STS ratings across study time points. Of these, 61 had a baseline and at least one post-baseline follow-up S-STS. Despite the small sample and low occurrence of suicidal ideation in this study, the authors found that the sensitivity of the S-STS (its ability to detect true cases of suicidal ideation or behavior) was very high compared to the rater-administered Hamilton Depression Rating Scale (HAM-D) item #3 (suicide) and in relation to reported suicidal adverse events (AEs) and suicidal serious adverse events (SAEs). The S-STS showed 100-percent sensitivity for identifying subjects with suicidal ideation and behaviors, compared to 63 percent for item 3 (suicide) on the HAM-D. These sensitivity calculations were based on any evidence of suicidal ideation or behaviors from review of AEs, SAEs, HAM-D item #3, or a S-STS score greater than 0.
Preti et al[15] evaluated the reliability (internal consistency and test-retest stability) as well as the convergent validity and divergent validity of the self-rated S-STS using a cross-sectional study in a nonclinical sample of 303 college students aged 18 to 40 years (mean age of 23.5 years). Within this sample, 29.4 percent had experienced passive suicidal ideation, 20.4 percent had experienced active suicidal ideation, 4.3 percent had planned a suicide attempt, 1.9 percent had engaged in preparatory suicidal behavior, 5.9 percent had engaged in self-injurious behavior without intent to kill themselves, and 2.6 percent had made a suicide attempt. Sixty subjects were contacted for retesting 4 to 6 weeks later, and 58 complied. Overall, the authors found “promising evidence on the convergent, divergent, internal consistency, and test-retest stability of the S-STS.”
Youngstrom et al[16] presented an analysis of the concordance of the S-STS with the C–SSRS in a clinical sample of 196 suicidal subjects drawn from inpatient and emergency room settings at Penn State Milton S. Hershey Medical Center in Hershey, Pennsylvania, USA. The analysis mapped each scale to the C-CASA categories that were adopted in the 2010 FDA Draft Guidance1 for suicidal assessment. The authors found acceptable concordance between the S-STS and the C–SSRS using Kappa scores. A further analysis using the updated 2012 FDA classification categories suicide ideation and behavior categories is underway by this group from this dataset.
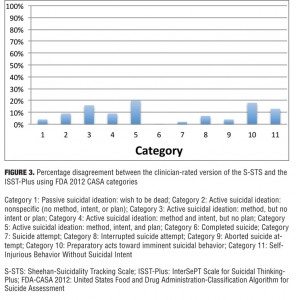
The University of Alabama at Birmingham (UAB) conducted a validation study comparing the S-STS and the ISST-Plus[17] with the C-SSRS (treated as the gold standard) in 40 adult subjects identified as having suicidal ideation or behavior with varying degrees of severity recruited from inpatient, outpatient, and emergency room settings. The data were analyzed using mapping to both the 2010 and the 2012 FDA Draft Guidances1,2 suicidality categories. The results were reported at five national scientific meetings in poster and oral presentations[11,18] and have generated one published article so far.[11] Subjects were rated on all three scales on the same day and were assigned to each scale in a random sequence order. The S-STS clinician-rated, S-STS patient-rated, and S-STS reconciliation versions were used in this study and were part of this validation analysis. In essence, the S-STS and ISST-Plus were very similar to each other in the agreement scores and diverged very little from each other, although the two scales appear different on face inspection and method of use and are derived from very different sources (Figure 3). However both the ISST-Plus and S-STS (in all 3 versions) showed marked differences with the C-SSRS on most of the suicidal ideation items, which are the essential core of most suicide assessments. The reasons for these differences are the subject of of another paper.[11] The problem here is the flawed navigation instruction for suicidal ideation on the C–SSRS relating to item 2, the flawed application of a Guttman Scaling procedure on the C–SSRS, and the related type I and type II errors on the C–SSRS that lead to under-endorsement of some suicidal phenomena and over-endorsement of others.[19] For example, the true rates of non-specific active suicidal ideation are inflated on the C-SSRS compared to reality. The Guttman Scaling assumption as used in the C–SSRS is not an optimal, comprehensive, reproducible, or generalizable assumption to model suicidal ideation and behavior. See Appendix H [CLICK TO ACCESS] for further details on why Guttman scaling is not optimal for use in the design of a suicidality scale. C-SSRS datasets cannot be merged with ISST-Plus and S-STS datasets because of these design flaws.
Evidence of reliability. To date, there are no published data supporting inter-rater reliability of the S-STS; however, the UAB clinical research team conducted a test-retest reliability and inter-rater reliability study in conjunction with the previously described validation study,[11] which confirmed the S-STS reliability. This study is currently being prepared into a manuscript for publication.[18]
Comparison of Clinician-rated, Self-report, and Reconciled Versions of the S-STS
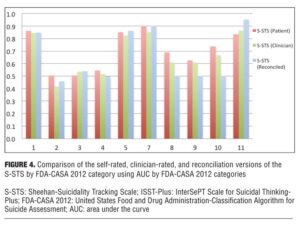
The correspondence between the clinician-rated, self-report (patient-rated), and reconciled versions of the S-STS appears to be high. As shown in Figure 4, the three versions showed similar patterns in the way they mapped or failed to map to the C–SSRS and the FDA-CASA 2012 categories in the UAB study described above.[11] The reconciliation version was more similar to the clinician-rated version than to the self-rated version, but the interpretation of this finding is complex. While it is tempting to assume that the clinician-rated and the reconciliation versions best reflect what actually occurred, one should not assume this is always or even usually the case.
Suicide Signal Detection Testing
There is a growing recognition of the need for signal detection in pharmacovigilance.[20] While the S-STS is used to detect suicidal signals as adverse events, it has also been used to identify an anti-suicidal efficacy signal in randomized, double-blind, controlled efficacy studies, including the study that compared BMS 562086, escitalopram, and placebo for treatment of GAD (as previously discussed);[13] a study that compared citalopram augmented by lithium to citalopram with placebo in a four-week study of suicidality in major depressive disorder (MDD), dysthymia, and depressive disorder not otherwise specificed (NOS);[21] and a study that examined a new investigational compound in pre-dementia Alzheimer’s disease.[22]
In the Coric et al study[13] comparing BMS 562086, escitalopram, and placebo (n=82, 61 with a post-baseline S-STS assessment) for treatment of GAD, there was a positive signal on the S-STS over eight weeks of treatment. A power analysis in this study showed that a sample size of 123 per treatment arm would be needed to detect this anti-suicidality efficacy signal for escitalopram on the S-STS at p<0.05 level in an adult GAD study. This sample size is less than the usual sample size per treatment arm in a typical multicenter clinical trial in central nervous system (CNS) research. It offers hope that the S-STS could be used as an efficacy signal detector in many CNS clinical trials searching for anti-suicidal effects from future investigation drugs.
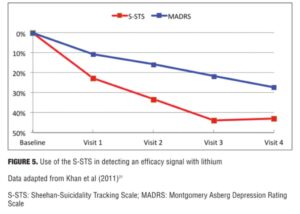
Khan et al[21] used the S-STS to study the anti-suicidal effects of citalopram augmented by lithium versus citalopram with placebo (n=80). The subgroup of patients on lithium with a blood level of 0.5mEq/L or higher showed significantly higher S-STS remission rates (45% compared to 19%, p<0.05). In this same study, the Beck Suicide Scale did not have adequate sensitivity to detect this signal at a p<0.05 level.
Figure 5 shows the percent symptom reduction in suicidal thoughts and behaviors and depressive symptoms as measured by the S-STS and the Montgomery Asberg Depression Rating Scale (MADRS) in the Khan et al[21] study. It shows that the anti-suicidal effects of lithium (as measured by the S-STS) can be disaggregated from any anti-depressant effects (as measured by the MADRS). According to Khan et al,[21] “The reduction of S-STS scores was large (43%) and twice that seen in MADRS scores (25%) among the 80 patients in the trial. Both response ([chi-squared]=8.8, p<0.01) and remission rates ([chi-squared]=4.6, p=0.03) showed similar patterns.”
Comparison of S-STS to C-SSRS. The S-STS differs from the C–SSRS in several respects. First, the C–SSRS uses a hierarchical Guttman-like scale structure for suicidal ideation items and combinations. As Mundt et al[12] observe, the presence of suicidal ideation on the C–SSRS is ascertained at five levels of increasing degrees of ideation severities. These include passive ideation and four levels of active ideation (thoughts of killing self, thoughts of method for killing self, intentions to kill self, and development of plans for committing suicide). The last three levels of ideation are only evaluated on the C–SSRS if thoughts of killing oneself are first endorsed.[12] Effectively this means that if the patient does not have non-specific, active suicidal ideation, the rater cannot inquire about method, plan, or intent. This is not the case with the S-STS. The S-STS does not assume nor make use of a Guttman scaling procedure or assumptions in evaluating suicidal ideation and or behaviors. Second, the C–SSRS uses a yes/no response format for the suicidal ideation items, whereas the S-STS allows finer tuned ratings on an ordered scale from 0 to 4. While the C–SSRS does provide for an intensity rating, it is categorical (not ordered) and only applies to the most severe category endorsed, based on its 1 to 5 hierarchical categorization. This means that other categories, even though they may be experienced as more severe, are not rated for intensity because they are ranked lower on this a priori hierarchy. Third, the C–SSRS is generally clinician-rated, whereas the S-STS can be patient-rated or clinician-rated.
Training, Current Use, and Applications
Training. The FDA requires training before suicidality instruments are used in clinical trials. Sponsors, rater training agencies, and clinical research organizations can handle the training on the S-STS as long as they adhere to the FDA’s expectations on the FDA-CASA 2012 definitions. Training is not required for licensed practitioners using the S-STS in clinical practice.
Current use. The most current data on usage of the S-STS in clinical trials comes from the 2013 International Society for CNS Clinical Trials and Methodology (ISCTM) survey. This survey assessed use of different suicidality instruments by 1,447 industry employees at 178 companies large and small. In a study examining these survey results, Chappell et al[23] received back 129 responses from 50 companies, but analyzed only the data on the subset of 86 respondents who indicated direct involvement with suicidal ideation and behavior. Results of the survey indicated that the C–SSRS was used for tracking suicidality by 94 percent of the participants, the S-STS by 22.4 percent, and the ISST-Plus by 10.4 percent. Some respondents noted that they had used more than one instrument.
In addition, the S-STS is currently being used in a national initiative assessing suicidality in South African Veterans mandated by the Parliament of South Africa.[24] The National Institute of Mental Health and Neuro Sciences (NIMHANS) in India is using an adapted version in its 2014 India national mental health epidemiology study.[25,26] It is difficult to get a precise estimate on the number of studies that have used the S-STS. The first author of this article (D.S.) has given permission for its use in many studies, including those investigating biomarkers and genetic linkage, where the S-STS disaggregated suicidal phenomena may provide the fine-grained phenotyping needed in such studies.
Applications. Careful suicidality assessment and tracking are needed and indeed increasingly mandated and have become routine in psychiatric research, mental health, and medical as well as other settings.[2]
Clinical trials. In clinical trials, the S-STS is a potentially powerful tool to detect anti-suicidal signals from new medications.[13,21,22] It also offers a valuable alternative to the C–SSRS for assessing suicidal ideation and behavior as an adverse event (AE) or serious adverse event (SAE).[13] As we observe in a companion paper, the S-STS captures a more complete range of suicidal ideation and behavior, reducing potential type II error (not capturing suicidal phenomena that do exist), and it is likely to have less type I error (identifying suicidal phenomena when they do not exist) because the S-STS avoids the flawed navigation instructions and hierarchical scaling assumptions inherent in the suicidal ideation section of the C–SSRS.[19]
Clinical practice. With increasing media attention to recent high profile suicides, incidents of “death by cop,” suicides associated with conjoint homicides or school killings, and the call for proper screening of those buying guns, suicide assessments cannot be haphazard. They need to be systematic, careful, and thoughtful, with the aim of helping the vulnerable in a humane, understanding way. The S-STS provides a useful screening and tracking tool in mental health, primary care, and emergency room settings. It allows clinicians to collect the documentation recommended in the prescribing information for most psychiatric medications for assessing and monitoring suicidality before and during the course of treatment. This serves both to protect patients and to protect clinicians and healthcare provider groups and institutions medico-legally. Unlike alternative scales, such as the C–SSRS, the S-STS can be self-rated on paper or by computer before each visit while the patient waits to see a clinician. This assists the clinician in routinely monitoring the patient’s suicidality.
Health maintenance and managed care organizations. Health maintenance and managed care organizations are taking increasingly active roles in suicide prevention to promote health and to contain costs associated with suicidal behaviors. The S-STS, with its sensitivity to signal detection and change,[13,21,22] has a potential role in this effort and can be integrated easily into an electronic medical record.
Epidemiology research. The S-STS has applications in national epidemiology studies to investigate national suicide statistics beyond the usual reporting of deaths by suicide. This may provide national health policy advisors with information to better plan and allocate funding in national efforts to reduce suicide. For example, S-STS is currently being used in adapted form, as noted above, in two major epidemiology initiatives—one in South Africa[24] and one in India (one of the largest epidemiology studies ever carried out in psychiatry[25,26]).
Military and military veterans. The increasing rate of suicide among members of the armed forces and veterans is another growing concern and has led to the implementation of programs to more carefully detect and monitor suicidality in these groups.[27] The ability to use the paper-based or electronic self-rated S-STS one page form provides a way to screen those from waiting lists with higher suicidality scores and to ensure they get higher priority and more urgent care.
Criminal justice. It is estimated that more than 400 suicides occur each year in local jails at a rate three times greater than in the general population, and, according to some estimates, suicide is now the third leading cause of death in prisons.[28,29] The S-STS, with its companion the Sheehan-Homicidality Tracking Scale (S-HTS),[30] is a potentially valuable tool for screening and follow-up of the incarcerated and those on parole. The S-STS can be used to detect suicidality and the S-HTS can detect both homicidality alone and combination homicidality/suicidality (e.g., murder-suicide) in these populations. The S-HTS is the only scale the authors are aware of that tracks these homicidal or homicidal/suicidal impulses, ideations, or behaviors with the necessary level of detail.
International security. The frequency of suicide terrorism is a growing concern internationally. While the determinants of suicide terrorist acts are complex, there is increasing evidence that antecedent suicidality may play a role in this phenomenon.[31] Surviving perpetrators are increasingly being apprehended and detained in hotspots, such as Israel, Palestine, Afghanistan, and Iraq, and some are being sent to rehabilitation programs (e.g., in programs in Saudi Arabia).[31] The third author of this article (I.S.) has called for international cooperation and sharing of information about this population as a preventative measure.[32] Consistent, systematic data collection on suicidality within this population using a tool such as the S-STS may be useful in detecting the extent to which antecedent and ongoing suicidality contributes to these acts. This could enable the international sharing and analysis of such data in order to better understand the suicide terrorist and to lead to better methods of prevention of such behavior. Because of its systematic collection of data[11] and its sensitivity to signal detection and change,[13,21,22] S-STS also has potential uses as a screening tool for victims of human security catastrophes, including war, famine, and displacement.
Schools and colleges. High profile suicides at schools and colleges/universities have alerted the public to the need for sensitive evaluation of students at all levels. As discussed earlier, as many as one fifth of college students have suicidal ideation, 4.3 percent had made a suicide plan, and 2.6 percent have made a serious suicide attempt.[15] The probability of a suicide attempt among subjects who had suicidal ideation and had made a plan captured on the self-rated S-STS was 46.2 percent.[15] Routine tracking of suicidality in this population when they come to the attention of college counselors is prudent, and the S-STS provides a useful tool for this purpose because it can be self-rated.
Concern about the need to monitor suicidality in vulnerable samples of children and adolescents, both on and off medications, persists. Indeed a recent study of automated healthcare claims from 11 health plans for 1.1 million adolescents, 1.4 million young adults, and 5 million adults from 2000 to 2010 found a significant, relative increase in psychotropic drug poisonings in adolescents and young adults since the FDA mandated boxed warnings on antidepressants used in this population.[33] The Pediatric versions of the S-STS have been linguistically validated for 6- to 8-year olds, 9- to 12-year olds, and 13- to 17-year olds, making them potentially useful tools for screening and tracking suicidality by school counselors.[9]
Conclusion
The S-STS has evolved to meet higher expectations for a safety and efficacy scale to assess and monitor suicidality in clinical, research, educational, and security settings. This update and the related appendices [CLICK TO ACCESS] should provide clinicians, researchers, and those charged with the responsibility to assess and monitor suicidality in institutional settings, simple and clear answers to frequently asked questions on the current (2014) status of the scale.
References
1. United States Food and Drug Administration, United States Department of Health and Human Services. Guidance for Industry: Suicidality: Prospective Assessment of Occurrence in Clinical Trials, Draft Guidance. September 2010. https://www.federalregister.gov/articles/2010/09/09/2010-22404/draft-guidance-for-industry-on-suicidality-prospective-assessment-of-occurrence-in-clinical-trials. Accessed October 1, 2014.
2. United States Food and Drug Administration, United States Department of Health and Human Services. Guidance for Industry: Suicidality: Prospective Assessment of Occurrence in Clinical Trials, Draft Guidance. August 2012. Revision 1. http://www.fda.gov/downloads/Drugs/Guidances/UCM225130.pdf. Accessed October 1, 2014.
3. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS) [online]. 2010. www.cdc.gov/injury/wisqars/index.html. Accessed October 1, 2014.
4. Neeleman J. A continuum of premature death: meta-analysis of competing mortality in the psychosocially vulnerable. Int J Epidemiol. 2001;30(1):154–162.
5. Baerger DR. Risk management with the suicidal patient: lessons from case law. Prof Psychol Res Pract. 2001;32:359–66.
6. Sheehan DV, Lecrubier Y, Harnett-Sheehan K, et al. The Mini International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview. J Clin Psychiatry. 1998;59(suppl 20):22–33.
7. Glossary. Health outcomes methodology. Medical Care. 2000;38(9 Suppl. II,II-7-II-13):2–10.
8. Wyrwich KW, Wolinsky FD. Identifying meaningful intra-individual change standards for health-related quality of life measures. J Eval Clin Pract. 2000;6:39–49.
9. Amado DM, Beamon DA, Sheehan DV. The linguistic validation of the Pediatric versions of the Sheehan-Suicidality Tracking Scale (S-STS). Innov Clin Neurosci 2014;11(9–10):141–163.
10. Posner K, Brown GK, Stanley B, et al. The Columbia–Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277.
11. Sheehan DV, Alphs L, Mao L. Comparative validation of the
S-STS, the ISST-Plus, and the
C-SSRS for assessing the suicidal thinking and behavior FDA 2012 cuicidality Categories. Innov Clin Neurosci 2014;11(9–10):32–46.
12. Mundt JC, Greist JH, Gelenberg A, et al. Feasibility and validation of a computer-automated Columbia-Suicide Severity Rating Scale using interactive voice response technology. J Psychiatr Res. 2010;44:1224–1228.
13. Coric V, Stock EG, Pultz J,
et al. Sheehan-Suicidality Tracking Scale (S-STS): preliminary results from a multicenter clinical trial in generalized anxiety disorder. Psychiatry (Edgmont). 2009;6(1):26–31.
14. Posner K, Oquendo MA, Gould M, et al. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164:1035–1043.
15. Preti A, Sheehan DV, Coric V, et al. Sheehan-Suicidality Tracking Scale (S-STS): reliability, convergent and discriminative validity in young Italian adults. Compr Psychiatry. 2013;54(7):842–849.
16. Youngstrom EA, Meyer R, Hameed A, Gelenberg A. Pennsylvania State study on the validation of the S-STS and the C-SSRS. Presented at 9th Annual Scientific Meeting. ISCTM. Washington, DC: February 19–21, 2013.
17. Lindenmayer JP, Czobor P, Alphs L, et al. The InterSePT scale for suicidal thinking reliability and validity. Schizophr Res. 2003;63:161–170.
18. Alphs L, Sheehan DV, Mao L, et al. Validation of the ISST-Plus, the
S-STS, and the C-SSRS for assessing suicidal thinking and behavior using mapping to the 2010 FDA Draft Guidance or C-CASA Categories. Poster presented at CNS Summit 2012. Boca Raton, FL: November 15–16, 2102.
19. Giddens JM, Sheehan KH, Sheehan DV. The Columbia-Suicide Severity Rating Scale (C-SSRS): Has the “Gold Standard” become a liability? Innov Clin Neurosci. 2014;11(9–10):66–80
20. Meyboom RHB, Egberts ACG, Edwards IR, et al. Principles of signal detection in pharmacovigilance. Drug Safety. 1997;16(6):355–365.
21. Khan A, Khan SR, Hobus J, et al. Differential pattern of response in mood symptoms and suicide risk measures in severely ill depressed patients assigned to citalopram with placebo or citalopram combined with lithium: role of lithium levels. J Psychiatr Res. 2011;45(11):1489–1496.
22. Coric V, Berman RM, Cedarbaum JM, Sheehan DV. Sheehan-Suicidality Tracking Scale in predementia Alzheimer’s disease. Presented at 9th Annual Scientific Meeting. ISCTM. Washington, DC: February 19–21, 2013.
23. Chappell P, Mahableshwarkar AR, Alphs L, et al. ISCTM Suicidal Ideation and Behavior Assessment Workgroup. Prospective assessment of suicidal ideation and behavior: an internet survey of pharmaceutical sponsor practices. Innov Clin Neurosci. 2014;11(9–10):14–22.
24. Soma Initiative. M.I.N.I. Mini International Neuropsychiatric Interview. http://www.soma-i.co.za/mini/default.asp. Accessed October 1, 2014.
25. National Institute of Mental Health and Neuro Sciences. http://www.nimhans.kar.nic.in/ Accessed October 1, 2014.
26. The Indian Express. Nimhans to conduct national mental health survey. http://indianexpress.com/article/india/india-others/nimhans-to-conduct-national-mental-health-survey/. Accessed October 1, 2014.
27. Kemp J, Bossarte R. Suicide Data Report: 2012. Department of Veterans Affairs, Mental Health Services, Suicide Prevention Program. http://www.va.gov/opa/docs/suicide-data-report-2012-final.pdf. Accessed October 1, 2014.
28. Hayes L. Suicide prevention in correctional facilities. In: Scott C, Gerbasi J (eds). Handbook of Correctional Mental Health. Washington, DC: American Psychiatric Publishing Inc.; 2005:69–88.
29. Mumola C. Suicide and Homicide in State Prisons and Local Jails. Bureau of Justice Statistics Special Report. Washington, DC: US Department of Justice, Office of Justice Programs, Bureau of Justice Statistics; 2005.
30. Sheehan IS. Professor Sheehan. Sheehan research scales. http://professorsheehan.com/scales/Accessed October 1, 2014.
31. Merari A. Driven to Death: Psychological and Social Aspects of Suicide Terrorism. Oxford, UK: Oxford University Press; 2010.
32. Sheehan IS. Are suicide terrorists suicidal? Innov Clin Neurosci. 2014;11(9–10):81–92.
33. Lu CY, Zhang F, Lakoma MD et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348-g3596.
(BMJ 2014;348:g3596 doi: 10.1136/bmj.g3596 (Published 18 June 2014).