
by Amer Cavalheiro Hamdan, PhD, and Mariana Drabik Vieira, MSc
Dr. Hamdan is with the Graduate Program in Psychology, Department of Psychology, Federal University of Parana in Curitiba, Brazil. Dr. Vieira is a psychologist, candidate to master’s in psychology, Department of Psychology, Graduate Program in Psychology, Federal University of Parana in Curitiba, Brazil.
Funding: No funding was provided for this study.
Disclosures: The authors have no conflicts of interest relevant to the contents of this article.
Innov Clin Neurosci. 2022;19(10–12):29–34.
Abstract
Background: Deep brain stimulation (DBS) is considered an alternative treatment for patients with rapidly developing Parkinson’s disease (PD). DBS can cause cognitive changes, and it is necessary to perform an executive assessment before and after DBS to better define the prognosis.
Objective: The aim of this study was to analyze the use of the Stroop test for assessment of cognitive functions in patients with PD undergoing DBS.
Methods: The systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Pubmed, Scopus, PsycInfo, and Web of Science were used as electronic databases. All included studies assessed the cognitive ability of patients with PD undergoing DBS through the application of the Stroop test.
Results: Thirty-five articles met the inclusion criteria. Among the studies, there were different formats of Stroop applications. Twenty-three articles presented negative results in relation to the individuals’ performances in Stroop, compared to the control groups. The results suggested that there was no correlation between low performance in the test and global cognitive risk for the patients.
Conclusion: Patients with DBS declined in Stroop performance and showed impairments in response inhibition and speed. These results are not related to the lack of cognitive security of DBS. The Stroop test can be combined with other cognitive instruments to ensure greater approximation of results with reality measures.
Keywords: Parkinson’s disease, deep brain stimulation, Stroop test
Deep brain stimulation (DBS) is a form of alternative treatment for patients with Parkinson’s disease (PD) in rapid development or who have complications arising from the use of medication, without improvement of motor symptoms.1 In DBS, stimulation is performed through an electrode implanted in the patient’s brain and is mainly placed in the subthalamic nuclei (STN) or globus pallidus interna (GPi),2 as well as other specific targets depending on the presented symptomatology. DBS is associated with improvement of motor symptoms, which contributes to improved quality of life of patients. However, data have shown alterations in cognitive functions, such as verbal fluency, executive functions, and memory, after implanting the devices.3 Considering that alterations in executive functions are commonly present in patients with PD, due to established connections between the prefrontal cortex and subcortical areas (basal ganglia) being directly affected by the disease,4 it is necessary to perform cognitive assessment before and after DBS for better prognostic definition.
There is heterogeneity in scientific studies regarding methodologies and instruments used to investigate executive functions in the population with PD, which makes it difficult to compare the results and scientific production.4 There is no single instrument to assess executive functions. The main tests used are the Wisconsin Card Test, Trail Making Test; Wechsler’s Scales; and Stroop test.5–7 The Stroop test was chosen for this review because of its wide use in assessment of executive functions in populations with PD.8
The Stroop test is an instrument for assessing executive functions and aims to measure an individual’s ability for selective attention, cognitive flexibility, and inhibition of automatic answers. The test has four stages: 1) read the name of the colors printed in black, 2) read the name of the colors printed in color, 3) name the color of colored squares, and 4) name the color in which the words are printed without reading the words. This last stage is called interference task.9 Many versions of the Stroop test have been developed over the years, and as such, the number of phases or items presented and application modes might change. The objective of this literature review is to analyze the use of the Stroop test in the assessment of cognitive functions of patients with PD undergoing DBS.
Methods
The systematic review was conducted in accordance the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) method.10 The searches were performed in the following electronic databases: a) PubMed (7/30/2020), with the descriptors “Parkinson’s disease” [MeSH] AND “Stroop” [MeSH] AND “deep brain stimulation,” with search filters “humans” and “English;” b) Scopus (07/30/2020), with the descriptors ALL (“Stroop test”) AND (“Parkinson’s disease”) AND (“deep brain stimulation”); c) PsycInfo (07/30/2020), with the descriptors “Stroop test” AND “Parkinson’s disease” AND “deep brain stimulation”, with the search filter “journal articles;” and d) Web of Science (07/30/2020), with the descriptors and search strategy: #1 (TS=(Stroop Test), #2 (TS=(Parkinson’s Disease), #3 (TS=(deep brain stimulation), #1 AND #2 AND #3. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO), number CRD42020196140.
Studies that assessed the cognitive ability of adults with PD undergoing DBS through the application of Stroop test were considered, with the inclusion criteria of studies published in English and cross, longitudinal, randomized, and control studies. Exclusion criteria were systematic reviews, opinions, and letters to the editor; full text studies unavailable for online access; studies that assessed cognition in PD after surgical intervention using an instrument other than the Stroop test; and studies that did not assess cognition.
In the first stage of article selection, two authors read the titles and abstracts independently, following the eligibility criteria. Then, the information referring to each study was extracted and organized in a table containing the following data: a) author names; b) characteristics of the sample; c) type of study; and d) main results related to the Stroop test. Discrepancies were resolved through consensus among researchers.
To assess the risk of bias of the studies, the articles were analyzed according to the Mixed Methods Appraisal Tool (MMAT).11 The objective of this tool is to assign a global quality score to the selected articles, according to the pre-established criteria.
Results
Thirty-five studies (n=35) out of 139 articles found in the referred databases met the inclusion and exclusion criteria for this literature review. The specifications are presented in Figure 1.
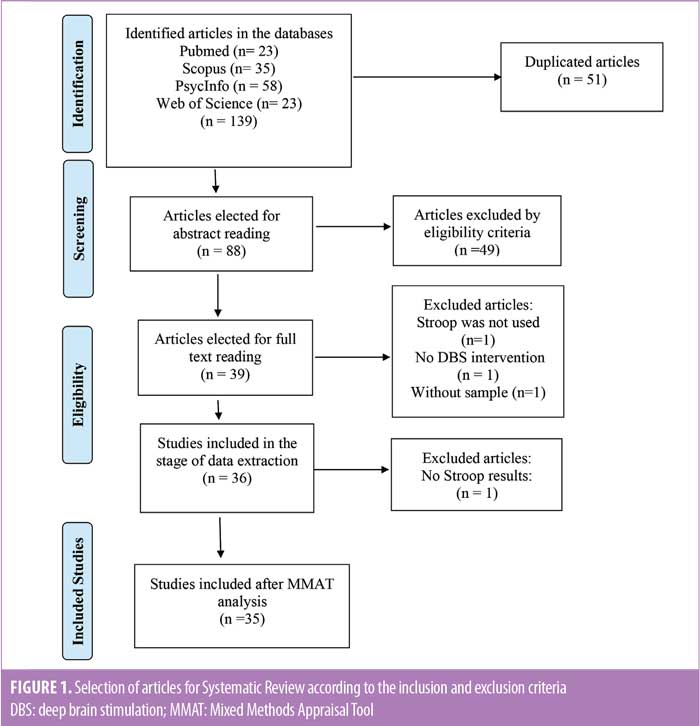
The main characteristics of each selected study are presented in Table 1. In general, most of the studies (n=21) were cohort research studies. Twelve studies used randomized samples, and two studies used a case-control research design. Seven out of 35 studies made comparisons between GPi and STN results, eight compared DBS stimulation with other types of drug treatments, 12 compared the performance of individuals in the Stroop test before and after surgery, and eight compared the performance of individuals with DBS and a control group consisting of individuals with PD without DBS. All the selected articles had the objective to assess or compare neuropsychological aspects associated with DBS.
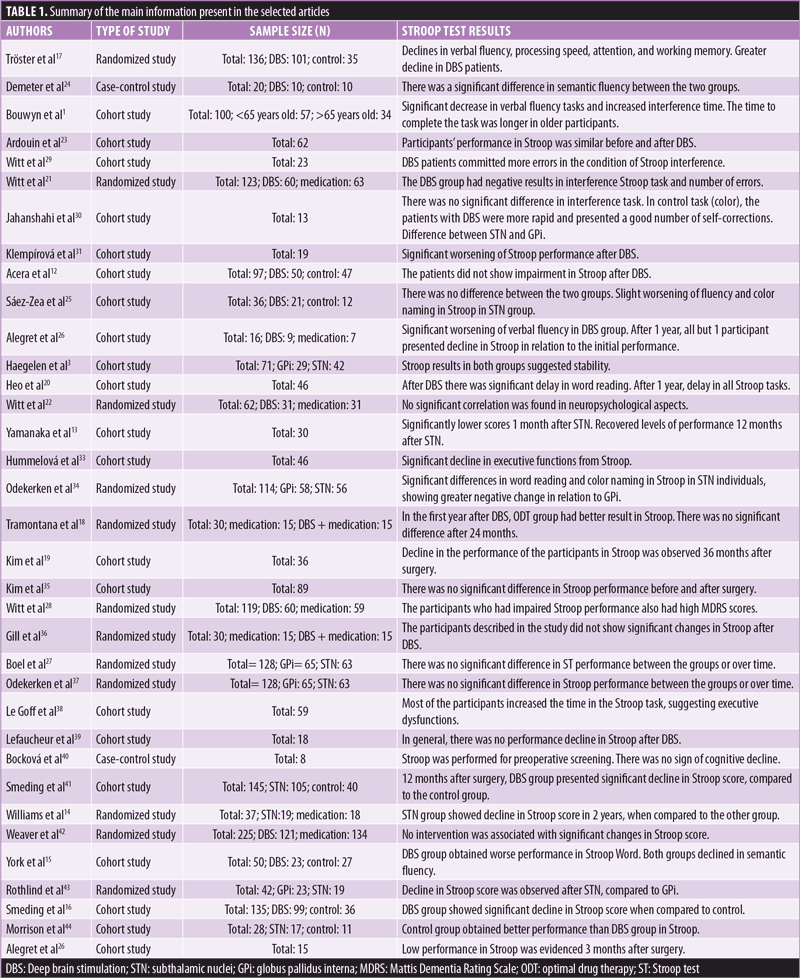
Regarding the applied versions of the Stroop test, the studies presented different formats of task application. Five studies described the Stroop application in four stages: “1) Word, 2) Color, 3) Color-word, and 4) Interference.” Five studies applied shorter or modified versions of the Stroop test, with one test containing only 36 items, for example. Most of the studies (n=18) used only two or three stages of Stroop, such as “1) Word, 2) Color, and 3) Color-word.” Finally, seven articles did not specify the Stroop application mode.
Considering the Stroop results for the population undergoing DBS, 23 studies (65%) presented negative performance in study participants, compared to the control groups, and 17 presented unfavorable results for the use of DBS for neuropsychological functioning. Ten studies presented positive results in relation to surgery performance, asserting that patients did not show significant cognitive impairment after the intervention. In general, the studies that presented cognitive decline in patients after DBS highlighted that, although surgical intervention improved quality of life and motor functions, declines in verbal fluency, processing speed, and executive functions, such as inhibitory control and memory, could occur. Eight studies presented inconclusive results about the cognitive effect of DBS.
Regarding the risk of bias of the selected articles, 20 of the selected studies obtained a score of 100 percent according to MMAT analysis criteria, 13 obtained a score of 75 percent, and two had scores below 75 percent.
Discussion
The objective of this article was to analyze the use of the Stroop test in patients with PD undergoing DBS. The results indicate declines in response inhibition and speed of task completion in the Stroop test. Although the instrument is commonly used and sensitive to analyze specific constructs of executive functioning, there was no consensus in the literature regarding the association between low performance in the Stroop test and the dimension of DBS impact on the global cognitive ability of the participants.
After surgery, individuals presented with a performance decline in the Stroop test. This result corroborates with existing studies in the literature that point to deficits in the postsurgical phase.12,13 In a study by Williams et al,14 patients with DBS declined in Stroop scores over the course of two years, mainly in the condition of interference, while the control group showed no alterations. Other studies15–18 found a decline in Stroop performance postintervention in all stages. Finally, Kim et al19 demonstrated that low performance in the Stroop test was significantly correlated to decline in global cognitive function.
Difficulties in the speed of information processing include delays in reading neutral words and naming colors, which was observed six months after surgery in a study by Heo et al.20 There were also deficiencies in self-correcting errors and prolonged reaction times.20 Reading time and error rates were significantly worse in the DBS group.21
In general, the condition of Stroop interference was the most-affected stage in the analysis of patient performance, which suggests difficulties in the control of attention and response inhibition. The hypothesis posed by Smeding et al16 was that stimulation can disable upper frontal cortex activity, which temporarily influences executive functioning. The electrical current from the stimulator can affect limbic areas, the hypothalamus, and other brain areas besides sensorimotor areas, which can have negative effects on the condition of Stroop interference.16 Other authors considered the factors of electrode position and stimulation range as related to negative results.22 Reduction of levodopa medication after surgery may also be a predicting factor of deterioration in Stroop performance.13,15,16
In disagreement with most of the results found in this review, the studies by Ardouin et al23 and Demeter et al24 showed that the performance of the participants in the Stroop test was similar before and after surgery. Sáez-Zea et al25 confirmed that DBS does not influence cognitive ability, contributing to the perspective that the decline observed in Stroop performance might be related to other factors, such as progression of PD and individual variables. In a study by Alegret et al,26 results showed that although DBS caused worse performance in the Stroop test, it did not produce clinically relevant neuropsychological deteriorations. These studies asserted that there was no direct or causal relation between DBS and cognitive declines observed after surgery.
It should be noted that there are differences in the neuropsychological constructs assessed by Stroop between the studies. In the research of Sáez-Zea et al,25 the test was used to exclusively measure participant attention. Boel et al27 used the Stroop test to assess measures of working memory, speed, and attention. In Witt et al,28 the test was used to assess attention and response inhibition. No pattern of application of the Stroop test was observed among the analyzed studies, which might affect the analysis and comparison of data. These variables can be better studied in future research in the area.
The Stroop test is one of the most widely used cognitive instruments in the evaluation of patients undergoing DBS.6,7 Unfavorable aspects regarding its use include the lack of standardization of the application and heterogeneity of the results, which can make its interpretation difficult. However, a favorable aspect is its quick and easy application, which helps its inclusion in clinical investigation protocols.
Although most of the individuals declined in Stroop performance after DBS, the results of this review suggests that there is no correlation between low performance in the test and global cognitive risk for patients. Despite presenting risk, which was temporary in many cases, to executive functions, DBS was considered safe from a cognitive perspective. Thus, it is possible to question the level of sensitivity of Stroop to assess an individual’s performance in daily activities and quality of life.
The importance of performing the cognitive assessment of patients who are candidates for DBS is the improvement in the quality of clinical care for this population. The Stroop test can be combined to other neuropsychological instruments, which compound standardized batteries, as well as ecological tasks that assess an individual’s functioning in daily activities. Thus, health professionals can work to prevent and/or reduce the impact of consequences generated by DBS in favor of improvements in quality of life.
Limitations. This study had some limitations. The first was the small number of databases for the selection of articles. Second, the descriptors used could have been more specific, since the number of excluded articles was high. The instrument used to assess the risk of bias in the articles could be more specific. Finally, this review did not distinguish between individuals with PD and individuals with PD and cognitive decline related to dementia or mild cognitive impairment, which might have interfered in the analysis of Stroop performance for this population.
For future studies using the Stroop test, experimental research and longer prospective studies are recommended, with standardized application of the Stroop test in a population with PD with DBS, with the objective to produce normative tables for this population and analyze the results inherent to the Stroop stages to find conclusions regarding instrument sensitivity in measuring the real cognitive impact on patients.
Conclusion
In summary, the results of this systematic review showed that individuals with PD who underwent DBS declined in Stroop test performance, presenting impairment in response inhibition and speed to perform a task. However, these results are not related to potential impacts on cognitive functions, which could be caused by DBS. The Stroop test can be an important tool to assess cognitive functions in patients with PD who undergo DBS.
Author Contributions
The study was developed by MDV and ACH. Searches, selection of articles, and review of eligibility were carried out by MDV and ACH. Data extraction and analysis were conducted by MDV and ACH. Critical evaluation of the results was performed by MDV and ACH. The article was written by MDV and ACH.
References
- Bouwyn JP, Derrey S, Lefaucheur R, et al. Age limits for deep brain stimulation of subthalamic nuclei in Parkinson’s disease. J Parkinsons Dis. 2016;6(2):393–400.
- Negida A, Elminawy M, El Ashal G, et al. Subthalamic and pallidal deep brain stimulation for Parkinson’s disease. Cureus. 2018;10(2):e2232.
- Haegelen C, Baumgarten C, Houvenaghel JF, et al. Functional atlases for analysis of motor and neuropsychological outcomes after medial globus pallidus and subthalamic stimulation. PLoS One. 2018;13(7):1–16.
- Macuglia GR, Rieder CR de M, Ameida RMM. Executive functions in Parkinson’s disease: literature review. Psycho. 2012;43(4):552–561.
- de Souza Arten TL, Hamdan AC. Executive functions in Parkinson’s disease with and without deep brain stimulation (DBS): a systematic review. Dement Neuropsychol. 2020;14(2):178–185.
- de Santana AN, Melo MRA, Minervino CAdSM. Instruments for the evaluation of executive functions: systematic review of the previous five years. Avaliação Psicológica. 2019:18(1):96–107.
- Romann AJ, Dornelles S, Maineri NL, et al. Cognitive assessment instruments in Parkinson’s disease patients undergoing deep brain stimulation. Dement Neuropsychol. 2012;6(1):2–11.
- Scarpina F, Tagini S. The Stroop color and word test. Front Psychol. 2017;8:557.
- Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests Administration, Norms, and Commentary, Third edition. Oxford University Press; 1991.
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1–9.
- Pluye P, Robert E, Cargo M, et al. Proposal: a mixed methods appraisal tool for systematic mixed studies reviews. 2011. http://mixedmethodsappraisaltoolpublic.pbworks.com/w/page/24607821/FrontPage. Accessed 15 Sep 2022.
- Acera M, Molano A, Tijero B, et al. Long-term impact of subthalamic stimulation on cognitive function in patients with advanced Parkinson’s disease. Neurologia. 2019;34(9):573–581.
- Yamanaka T, Ishii F, Umemura A, et al. Temporary deterioration of executive function after subthalamic deep brain stimulation in Parkinson’s disease. Clin Neurol Neurosurg. 2012;114(4):347–351.
- Williams AE, Arzola GM, Strutt AM, et al. Cognitive outcome and reliable change indices two years following bilateral subthalamic nucleus deep brain stimulation. Park Relat Disord. 2011;17(5):321–327.
- York MK, Dulay M, Macias A, et al. Cognitive declines following bilateral subthalamic nucleus deep brain stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008;79(7):789–795.
- Smeding HMM, Speelman JD, Koning-Haanstra M, et al. Neuropsychological effects of bilateral STN stimulation in Parkinson’s disease: a controlled study. Neurology. 2006;66(12):1830–1836.
- Tröster AI, Jankovic J, Tagliati M, et al. Neuropsychological outcomes from constant current deep brain stimulation for Parkinson’s disease. Mov Disord. 2017;32(3):433–440.
- Tramontana MG, Molinari AL, Konrad PE, et al. Neuropsychological effects of deep brain stimulation in subjects with early stage Parkinson’s disease in a randomized clinical trial. J Parkinsons Dis. 2015;5(1):151–163.
- Kim HJ, Jeon BS, Yun JY, et al. Initial cognitive dip after subthalamic deep brain stimulation in Parkinson disease. J Neurol. 2013;260(8):2130–2133.
- Heo JH, Lee KM, Paek SH, et al. The effects of bilateral Subthalamic Nucleus Deep Brain Stimulation (STN DBS) on cognition in Parkinson disease. J Neurol Sci. 2008;273(1–2):19–24.
- Witt K, Daniels C, Reiff J, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol. 2008;7(7):605–614.
- Witt K, Granert O, Daniels C, et al. Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson’s disease: results from a randomized trial. Brain. 2013;136(7):2109–2119.
- Ardouin C, Pillon B, Peiffer E, et al. Bilateral subthalamic or pallidal stimulation for Parkinson’s disease affects neither memory nor executive functions: a consecutive series of 62 patients. Ann Neurol. 1999;46(2):217–223.
- Demeter G, Valálik I, Pajkossy P, et al. The effect of deep brain stimulation of the subthalamic nucleus on executive functions: impaired verbal fluency and intact updating, planning and conflict resolution in Parkinson’s disease. Neurosci Lett. 2017;647:72–77.
- Sáez-Zea C, Escamilla-Sevilla F, Katati MJ, Mínguez-Castellanos A. Cognitive effects of subthalamic nucleus stimulation in Parkinson’s disease: a controlled study. Eur Neurol. 2012;68(6):361–366.
- Alegret M, Junqué C, Valldeoriola F, et al. Effects of bilateral subthalamic stimulation on cognitive function in Parkinson disease. Arch Neurol. 2001;58(8):1223–1227.
- Boel JA, Odekerken VJJ, Schmand BA, et al. Cognitive and psychiatric outcome 3 years after globus pallidus pars interna or subthalamic nucleus deep brain stimulation for Parkinson’s disease. Park Relat Disord. 2016;33:90–95.
- Witt K, Daniels C, Krack P, et al. Negative impact of borderline global cognitive scores on quality of life after subthalamic nucleus stimulation in Parkinson’s disease. J Neurol Sci. 2011;310(1–2):261–266.
- Witt K, Pulkowski U, Herzog J, et al. Deep brain stimulation of the subthalamic nucleus improves cognitive flexibility but impairs response inhibition in Parkinson disease. Arch Neurol. 2004;61(5):697–700.
- Jahanshahi M, Ardouin CMA, Brown RG, et al. The impact of deep brain stimulation on executive function in Parkinson’s disease. Brain. 2000;123(6):1142–1154.
- Klempírová O, Jech R, Urgosík D, et al. Deep brain stimulation of the subthalamic nucleus and cognitive functions in Parkinson’s disease. Prague Med Rep. 2007;108(4):315–323.
- Alegret M, Valldeoriola F, Martí MJ, et al. Comparative cognitive effects of bilateral subthalamic stimulation and subcutaneous continuous infusion of apomorphine in Parkinson’s disease. Mov Disord. 2004;19(12):1463–1469.
- Hummelová Z, Baláž M, Janoušová E. Preoperative visual memory performance as a predictive factor of cognitive changes after deep brain stimulation of subthalamic nucleus in Parkinson’s disease. Cesk Slov Neurol N. 2016;79/112(6):680–686.
- Odekerken VJJ, Boel JA, Geurtsen GJ, Schmand BA. Neuropsychological outcome after deep brain stimulation for Parkinson disease. Neurology. 2015;85(16):1433–1434.
- Kim YE, Kim HJ, Kim HJ, et al. Impulse control and related behaviors after bilateral subthalamic stimulation in patients with Parkinson’s disease. J Clin Neurosci. 2013;20(7):964–969.
- Gill CE, Allen LA, Konrad PE, et al. Deep brain stimulation for early-stage Parkinson’s disease: an illustrative case. Neuromodulation. 2011;14(6):515–522.
- Odekerken VJJ, Boel JA, Schmand BA, de Haan RJ. GPi vs STN deep brain stimulation for Parkinson disease: three-year follow-up. Neurology. 2016;87(7):745–746.
- Le Goff F, Derrey S, Lefaucheur R, et al. Decline in verbal fluency after subthalamic nucleus deep brain stimulation in Parkinson’s disease: a microlesion effect of the electrode trajectory? J Parkinsons Dis. 2015;5(1):95–104.
- Lefaucheur R, Derrey S, Martinaud O, et al. Early verbal fluency decline after STN implantation: is it a cognitive microlesion effect? J Neurol Sci. 2012;321(1–2):96–99.
- Bočková M, Chládek J, Jurák P, et al. Involvement of the subthalamic nucleus and globus pallidus internus in attention. J Neural Transm. 2011;118(8):1235–1245.
- Smeding HMM, Speelman JD, Huizenga HM, et al. Predictors of cognitive and psychosocial outcome after STN DBS in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2011;82(7):754–760.
- Weaver FM, Stern M, Harris C, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson’s disease. 2009;301(1):63–73.
- Rothlind JC, Cockshott RW, Starr PA, Marks WJ. Neuropsychological performance following staged bilateral pallidal or subthalamic nucleus deep brain stimulation for Parkinson’s disease. J Int Neuropsychol Soc. 2007;13(1):68–79.
- Morrison CE, Borod JC, Perrine K, et al. Neuropsychological functioning following bilateral subthalamic nucleus stimulation in Parkinson’s disease. Arch Clin Neuropsychol. 2004;19(2):165–181.


