
by Shama Akram, MPhil; Moazzam Ali, PhD; Zeeshan Mutahir, PhD; Nabeel Ibad, FCPS; Sana Sarmad, MPhil; Sumaira Mehboob, PhD; and Mahjabeen Saleem, PhD
Ms. Akram and Drs. Ali, Mutahir, and Saleem are with School of Biochemistry and Biotechnology, University of the Punjab in Lahore, Pakistan. Dr. Ibad is with Shaikh Zayed Hospital in Lahore, Pakistan. Dr. Sarmad is with Rashid Latif Medical College in Lahore, Pakistan. Dr. Mehboob is with School of Biochemistry, Minhaj University Lahore in Lahore, Pakistan.
Funding: This work was supported by University of the Punjab in Lahore, Pakistan.
Disclosures: The authors have no conflicts of interest relevant to the contents of this article.
Innov Clin Neurosci. 2023;20(1–3):60–71.
Abstract
Objective: Gene-environment interactions might play a significant role in the development of bipolar disorder (BD). The objective of the current study was to investigate the association between tumor necrosis factor (TNF)-α -308 G/A polymorphism and BD and conduct a bioinformatics analysis of the protein-protein network of TNF-α. Gene-environment interactions and the relationship between stressful life events (SLEs) and substance abuse with TNF genotypes and other characteristics were analyzed.
Methods: The genomic deoxyribonucleic acid (DNA) of 400 patients with BD and 200 control subjects were extracted and genotyped for TNF-α -308 G/A polymorphism. SLEs and substance abuse were evaluated using the Life Event and Difficulty Schedule (LEDS) and a self-designed substance abuse questionnaire for the events six months prior to the onset of the disease, respectively. Gene-environment interactions were assessed by multiple statistical tools. Bioinformatics analysis of the TNF-α network and its interacting proteins was carried out using STRING and Cytoscape softwares.
Results: Genotyping analysis revealed a significant association between TNF-α -308 G/A polymorphism and BD (p<0.009). Furthermore, analysis of gene-environment interaction revealed a significant association between TNF-α -308 G/A and SLEs (p=0.001) and TNF-α -308 G/A and substance abuse (p=0.001). Three distinct proteins, RELA, RIPK1, and BIRC3, were identified through hub analysis of the protein network.
Conclusion: TNF-α -308 G/A polymorphism is positively associated with BD. SLEs and substance abuse might trigger the early onset of BD. Proteins identified through bioinformatics analysis might contribute to the TNF-α mediated pathophysiology of BD and can be the potential therapeutic targets.
Keywords: Bipolar disorder, TNF-α, polymorphism, stressful life event, inflammation
Bipolar disorder (BD) is a mental illness, previously known as manic-depressive illness or manic depression.1 Globally, it is the sixth-leading cause of disability and is a health concern since, despite receiving treatment, symptoms recur in nearly half of all patients with the disorder.2 For about half of their lifespan, patients with BD suffer from manic and depressive symptoms of a cyclic nature, which is a major cause of disability in these patients.3 Social, economic, and quality of performance impairments, as well as increased risk of suicidal behavior and other comorbidities, are also commonly reported in the patients.4
Although much investigation has been undertaken to understand the pathophysiology of BD, the underlying mechanisms and biology still remain to be explored.5 Low-grade inflammation has been associated with BD, making the role of the immune system an area of great interest among researchers.6,7 The neurobiological mechanisms that contribute to impaired neurodevelopment in BD are still unclear, but inflammation seems to be the major contributor.8 It has been suggested that inflammatory cytokines, particularly tumor necrosis factor (TNF), might play a crucial role in the pathogenesis of BD by controlling the activity of immune cells and increasing the secretion of other proinflammatory cytokines.9 In the event of some injuries or infections, TNF-α is secreted by macrophages, neutrophils, lymphocytes, and other immune cells.10 TNF-α is also produced from certain structural cells, such as astrocytes and neurons, exerting pleiotropic effects on neural development and neural transmission.11 TNF-α acts on TNF receptor 1 (TNFR1), activates caspases and apoptotic factors, and can induce neuronal cell death; thus, TNF-α might be related to neuronal and glial loss in BD.12
BD has a strong genetic predisposition. Multiple genetic alterations, such as insertion, deletion, and duplication, might play roles in the development of BD, but the most common genetic alteration is single nucleotide polymorphism (SNP).13 SNP is a change in a single nucleotide of deoxyribonucleic acid (DNA) that can alter the expression of a gene.14 In the promoter region of TNF-α, multiple polymorphisms have been associated with changes in the circulating levels of TNF-α.15 TNF-α −308G/A promoter polymorphism (rs1800629) at the nucleotide position −308 is one of the most important SNPs, due to its effect on the binding of the transcription factor to the consensus sequence.16 TNF-α -308G/A polymorphism is a base exchange polymorphism, creating a less common allele A at position -308, which has been associated with greater TNF-α production and rate of TNF-α transcription than that associated with the wild-type GG genotype.17 This uncommon TNF-α allele has been associated with higher morbidity and mortality in several immune and infectious disorders, including psoriasis, arthritis, vascular and neurological diseases.18
In addition to genetic involvement, several social and environmental factors might contribute to the pathophysiology of BD.19 Stressful life events (SLEs) might trigger the onset of BD and lead to hospitalization of the patients.20 Any chronic stressor or event could lead to relapse or recurrence of bipolar symptoms, which is a crucial issue in this disease.21 Genetic traits of a person interact with the environmental factors (i.e., gene-environment [G × E] interaction), leading to the onset or progression of any disease, especially psychological illness.22
Stress can mediate immune response and provoke the release of proinflammatory mediators, such as interleukin (IL)-1, IL-6, and TNF-α. It has been reported that blood levels of TNF-α increase in multiple diseases related to stress23 and various mental illnesses, such as major depressive disorder,24 schizophrenia,25,26 and BD.10,27 Elevated levels of TNF-α have been associated with SLEs, but the link between TNF-α polymorphism and SLEs has not been established.28
Comorbid substance abuse is one of the many risk factors with BD.29 There are two potential reasons for this risk: either patients with BD are more prone to substance abuse,30 or substance abuse triggers early-onset BD in susceptible.31 Substance use in BD might lead to violent behavior, increased suicidal behavior, nonadherence to medication, delayed recovery from mood episodes, and poor quality of life.29 Proper identification of risk factors can help providers to plan an effective therapeutic strategy for each patient. Rehabilitation of patients with BD and substance abuse will help them to adhere and respond to treatment effectively.
The main aim of this cross-sectional investigation was to verify an association between TNF-α -308G/A polymorphism and the predisposition of BD in the local Pakistani population. Another objective was to explore G × E interaction. The correlation between risk factors, such as SLEs and substance abuse, with the onset of BD and TNF-α -308G/A polymorphisms was observed. Furthermore, by using in-silico bioinformatics tools, new target proteins were analyzed that might help to understand the pathology of the disease.
Materials and Methods
Selection of study group. A total of 400 patients with BD (272 male and 128 female) with a mean age of 38.26±12.20 years were registered for this cross-sectional study. Patients were recruited from the psychiatric departments of three Pakistani hospitals. Hospitalized patients as well as those treated on an outpatient basis were included in this study. BD diagnosis was confirmed by two senior certified psychiatrists using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) and other available information (e.g., other clinician reviews, previous clinical reports, and information from family members). Patients over the age of 17 years were included, and patient age ranged from 17 to 69 years. Clinical observations, family history, age of onset, number of hospitalizations, presence of psychotic features, rapid cycling of mood, suicidal behavior, cognitive behavior, history of substance abuse, and other demographic characteristics were recorded in the questionnaire designed for this study. Subjects under the age of 17 years, with dementia, any neurological illness, comorbid mental illness, or any autoimmune disease were excluded from the study.
For the control group, 200 healthy subjects (130 male and 70 female) with a mean age of 36.56±10.87 years from the same demographic area were selected. The controls also underwent an interview to confirm the absence of any severe psychiatric illness, family history of psychiatric disease, history of neurological illness, endocrine disorders, and autoimmune disease. The purpose and procedure of the study were clearly explained to every patient and control subject, and written signed consent was received from each participant. Approval from the ethical board of the University of the Punjab in Lahore, Pakistan, was given prior to the commencement of the study. Approval from the concerned hospital authority was granted for sample collection.
Genotyping. Three-milliliter venous blood samples were collected in ethylenediaminetetraacetic acid (EDTA)-containing vacutainers (Vacuette, Greiner Bio-One; Frickenhausen, Germany) from patients and control subjects after their consent.
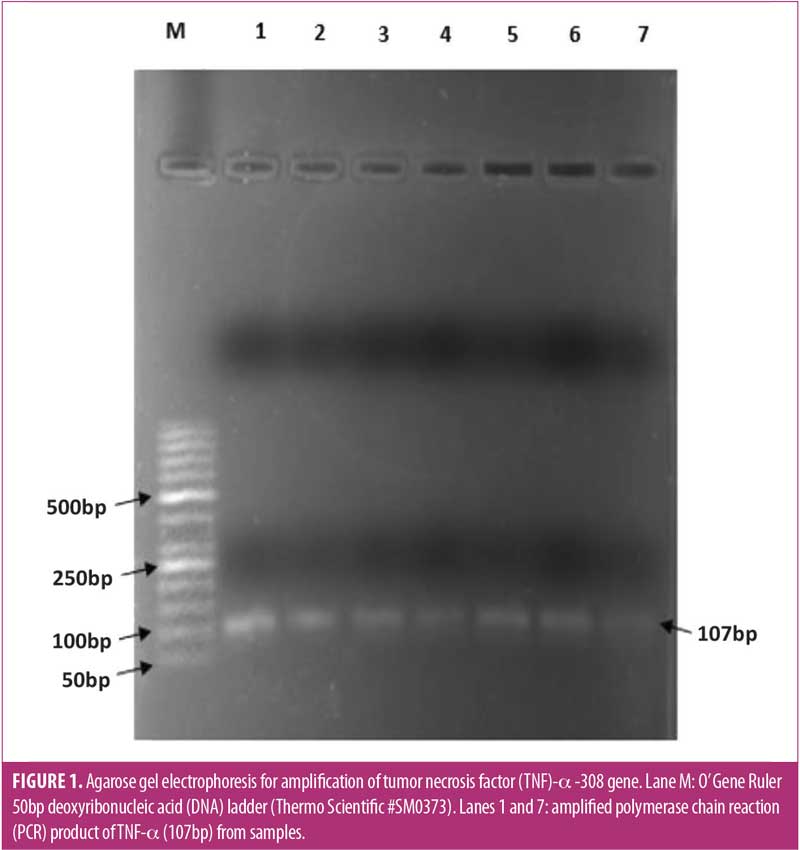
Genomic DNA was extracted from the blood through GeneJET genomic DNA purification kit (Thermo Fisher Scientific; Waltham, Massachusetts, United States) using the provided standard protocol. Genotyping of TNF-α -308G/A promoter polymorphism (rs1800629) was carried out using polymerase chain reaction, restriction fragment length polymorphism (PCR-RFLP). Oligonucleotide primers 5’ -AGGCAATAGGTTTTGAGGGCCAT-3’ (forward) and 5’-TCCTCCCTGCTCCGATTCCG-3’ (reverse) were used to amplify 107bp TNF-α PCR product (T100 Thermal Cycler, Bio-Rad; Hercules, California, United States). A 25µL PCR amplification mixture contained 150 to 300ng of genomic DNA, 0.5µm of each primer, 0.2mM of each deoxynucleoside triphosphate (dNTP), 1.5mM magnesium chloride, 75mM Tris hydrochloride, 20mM ammonium sulfate, 0.01 percent (v/v) polysorbate 20, and 0.5 units of Taq DNA polymerase (Thermo Fisher Scientific; Waltham, MA, US). Cyclic conditions were initial denaturation at 95C° for five minutes, 35 cycles, with a profile of 95C° for 30 seconds, 55C° for 45 seconds, and 72C° for 45 seconds, followed by final elongation at 72C° for 10 minutes. For restriction analysis, 10µL of each PCR product was digested overnight in a total volume of 18µL at 37C° with 0.5 units of NcoI restriction endonuclease (Thermo Fisher Scientific; Waltham, Massachusetts, United States). Afterward, digestion products were subjected to 3% agarose gel electrophoresis and visualized by ethidium bromide staining. Band sizes were compared with a 20bp DNA ladder (Thermo Fisher Scientific; Waltham, Massachusetts, United States).
Evaluation of SLEs. All subjects registered in the study were interviewed using the Life Events and Difficulty Schedule (LEDS).32 The LEDS is a semistructured questionnaire for the assessment of life events and prolonged effect of difficulties in adults.
To estimate the effect of life events on the onset of BD, the recall period was limited to six months. All the patients were asked to relay any SLE within six months before the onset of first mood disturbance incident. Onset is defined as the duration in which first affective incident, according to DSM-IV criteria (depressive, manic, mixed, oy hypomanic) occurred. For controls, any SLE six months prior to the interview were recorded. Two trained research assistants conducted the LEDS interview with all participants. They confirmed that the event occurred in the index period and acquired contextual information to determine whether the reference event satisfied the classification of items. The LEDS encompasses maximum types of stressful episodes associated with education, job, illness, bereavement (immediate family or close relative or friend) marital/relationship break-up, conflict with close friend or relative, childbirth, legal issues, and theft or loss. After consideration of contextual information, each event was analyzed and rated through standardized rating protocols for its threat level. Threat score depicted the severity of the event, ranging from mild (1) to severe (4), so that mild and severe life events were differentiated from each other. Events were rated by two independent raters who had not been involved in the interviews. Many studies have supported the validity and reliability of the LEDS in adults.33
Evaluation of substance abuse. Previous research has shown that patients with BD are at increased risk of substance abuse.34 History of substance abuse was evaluated in our study patients with BD with a self-designed questionnaire in which substance use history six months prior to the interview was recorded. The interview was carried out by trained research assistants. Patients were asked about the age at which they first used a substance. The frequency of substance use and type of substance used was also documented. As none of the control subjects experienced substance abuse, we divided patients with BD in two groups, those with and without substance abuse, and compared their clinical and demographic characteristics, as well as TNF-α -308G/A, using statistical analysis.
Statistical analysis. Statistical analysis was carried out using SPSS for Windows version 25. Genotype and allele frequencies of the patient groups and controls were examined using the standard Chi-squared test. Probability (p) values of less than 0.05 were considered statistically significant. The odds ratio (OR) with respective confidence interval (95% CI) for disease susceptibility was also calculated. The observed frequency of polymorphism between patients and controls was tested for Hardy–Weinberg equilibrium (HWE) using the v2 method. Group differences were tested for categorical variables using Chi-squared and Fisher’s exact test, while t-test was used to analyze interval scale or ordinal data. Logistic regression was applied to predict the interaction of family history, SLEs, and substance abuse with clinical and functional characteristics.
For G × E interactions, study recommendations from Uher et al35 were followed. The main effects and interactions between SLEs and TNF-α -308G/A on the onset of bipolar disorder were analyzed using a generalized linear model with binomial distribution to estimate the risk differences (RDs) with 95% CIs. Two genetic models were tested: additive (0=AA, 1=GA, 2=GG) and GG versus A carriers (GA and AA=0, GG=1). As AA genotype is rare, TNF-α -308G/A genotype was sorted into GG and A carriers (i.e., GA and AA), as previously used by other researchers also examining G × E interactions.36 These interaction analyses were conducted through STATA version 14.
Bioinformatics analysis. To construct a protein-protein interaction (PPI) network of TNF-α, we used the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) v11.0 bioinformatics database (https://string-db.org/) to compute the known and predicted proteins interacting with TNF.37 Interactions related to Homo sapiens only were analyzed, and the number of interactors in the first shell was restricted to 50. The minimum required interaction score was set to the highest confidence (0.900) to obtain true interactions and avoid imprecise interactions. It ensured that more than 80 percent of these interactions would probably be replicated in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.38 The interacting proteins were further arranged into five clusters based on their similarity or relatedness, using the k-mean algorithm provided by the STRING database. The network was exported to Cytoscape v3.8.2.39 The hubness of the network was analyzed using the cytoHubba plugin in Cytoscape bioinformatics software.40 We selected two ranking methods (i.e., degree and betweenness) to identify biologically related significant proteins. Due to their strong interactions, these proteins might play an essential role in BD.
Results
Demographic features of the patients. Out of 400 patients with BD, 128 (32%) were female and 272 (68%) were male. The control group comprised 70 (35%) female and 130 (65%) male participants. Overall, the mean duration of illness was 11.17 years (standard deviation [SD]±11.13), mean age of onset of disease was 27.18 years (SD±7.32), average number of manic episodes was 4.42 (SD±4.12), and average number of depressive episodes was 5.30 (SD±4.51). Demographic and clinical characteristics of cases and controls are shown in Table 1.
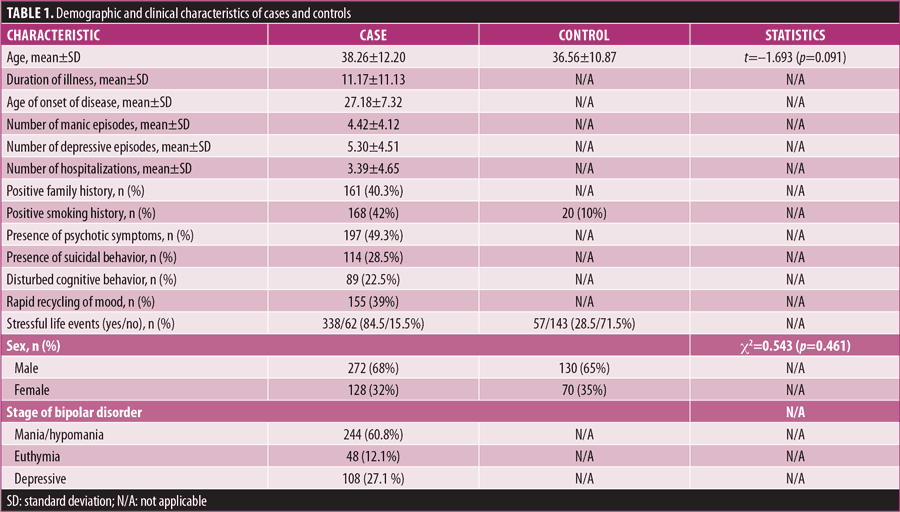
Among male patients, 166 had mania/hypomania, 40 were euthymic, and 66 had depressive symptoms. Among female patients, 78 had mania/hypomania, 8 were euthymic, and 42 had depressive symptoms.
Family history and BD. Previous reports indicate that people with a family history of mood disorders, especially BD, in a first-degree relative are at a greater risk to develop that disorder, compared to those with no family history of mood disorder.41 To assess the effect of family history on the severity of the associated symptoms, we divided the patients into two groups: those with positive family history of mood disorders and those without family history of mood disorders. We did not take controls into consideration during this analysis since positive family history of mental illness was an exclusion criterion. The comparative analysis is shown in Table 2. There was no significant difference in age of onset of BD (p=0.517), number of hospitalizations (p=0.766), presence of psychotic symptoms (p=0.885), or disturbed cognitive behavior between patients with positive family history and those without family history. There was evidence of increased duration of illness (p=0.024) among patients with positive family history of mood disorders. Taking into consideration history of smoking and substance abuse, it was found that those patients without a positive family history of mood disorders were more likely to smoke (p=0.016) and experience substance abuse (p=0.022).
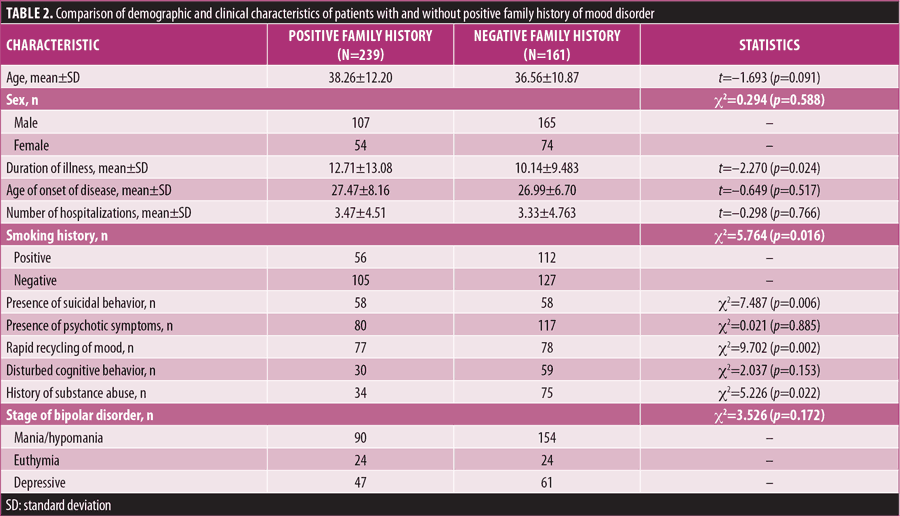
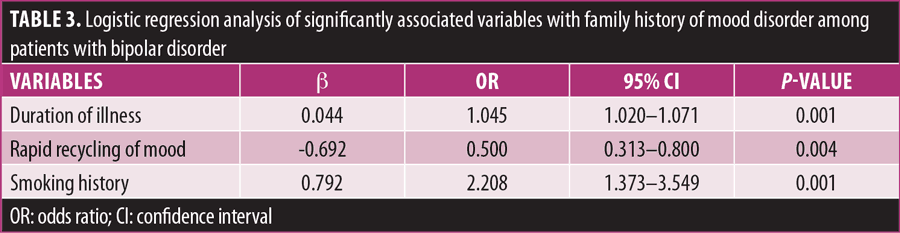
Stepwise binary logistic regression was applied to authenticate the association of symptoms with family history of mood disorder among patients with BD (Table 3). It was confirmed that duration of illness (p=0.001) and rapid recycling of mood (p=0.004) among patients with BD were significantly associated with positive family history of mood disorder, while the rest of the clinical demographics did not show significant association with family history. According to regression analysis, substance abuse lost its significance, while smoking history among patients with BD remained significantly related to positive family history of BD (p=0.001).
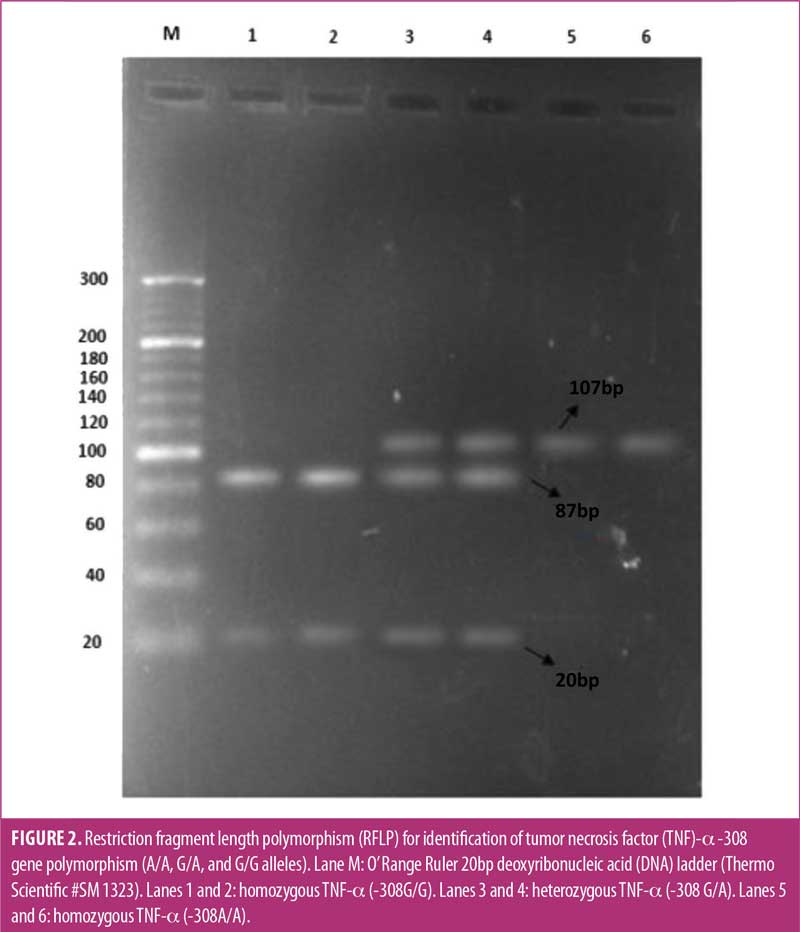
Genotyping analysis. Amplification of a specific region of TNF-α -308 yielded a 107bp fragment (Figure 1). After enzymatic digestion of the PCR product with NcoI restriction enzyme and incubation at 37C° for 16 hours, the TNF-α -308G/A PCR-RFLP products were visualized on the agarose gel. Homozygous TNF-α -308G/G genotype appeared as two fragments of 87 and 20bp, heterozygous TNF-α -308G/A appeared as three fragments of 107, 87, and 20bp, and homozygous TNF-α -308A/A appeared as an uncut 107bp fragment (Figure 2).
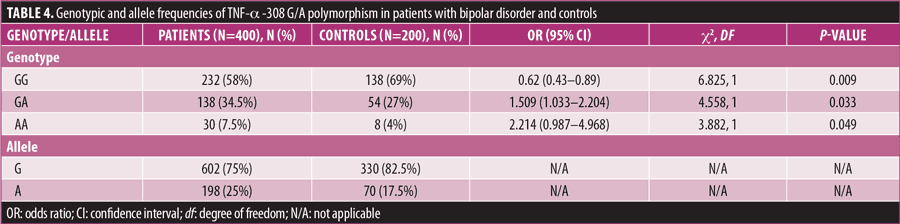
The genotypic and allele frequencies of TNF-α -308G/A polymorphism in patients with BD and controls are shown in Table 4. OR with a 95% CI was also determined. P-value of less than 0.05 was considered significant. The genotype and allele frequencies did not deviate from the HWE.
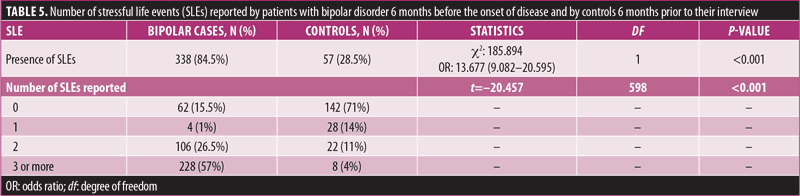
SLE and onset of BD. To explore the importance of G × E interaction in the development and progress of BD, the relationship between SLEs and TNF-α genetic polymorphism was investigated. A total of 84.5 percent of patients with BD experienced a stressful episode before the onset of BD, while 15.5 percent did not experience any SLE prior to the onset of BD. Thus, the onset of BD was greatly influenced by SLEs (p≤0.001, Table 5). Significantly more SLEs were reported six months before the onset of symptoms by patients with BD, compared to SLEs reported by controls six months prior to the interview (p≤0.001). There were five major life events that were frequently reported by patients with BD six months prior to the onset of symptoms. The most frequent SLE reported was a serious problem with a close friend, neighbor, or relative (including spouse), which affected 48 percent of the patients. Other SLEs reported were serious illness, injury or assault to the patient (30%), death of a close family friend or relative (25%), financial crisis (20%), and separation or marital difficulties (17%).
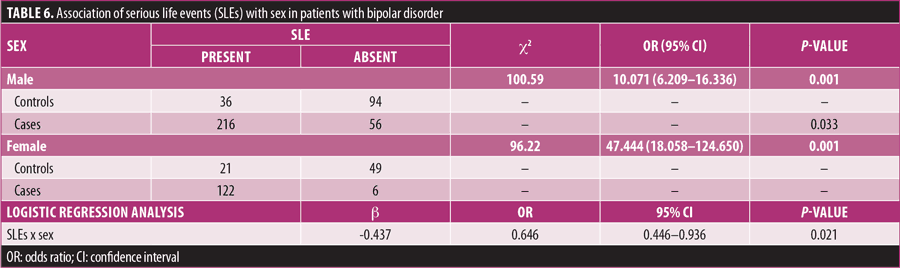
A significant association of sex with SLE was found when assessed through Chi-squared test (p=0.001), and the effect of SLE remained significant when analyzed through binary logistic regression (p= 0.021, Table 6). OR demonstrated that SLE can be a significant triggering factor to initiate bipolar symptoms in female patients, compared to male patients. The most frequently reported SLE by male patients was financial insufficiency and family crisis, whereas the most frequently reported SLE by female patients was stressed relationship and mental distress.
SLE and TNF-α -308G/A genotype (G × E interaction). The main and interaction effects of TNF-α -308G/A and SLEs are reported in Table 7. Both the additive model and GG versus A carrier model were tested and showed a significant main effect. SLEs prior to onset of disease depicted a strong main effect and were significantly associated with BD (RD: 0.74, 95% CI: 0.62–0.85, p≤0.001).
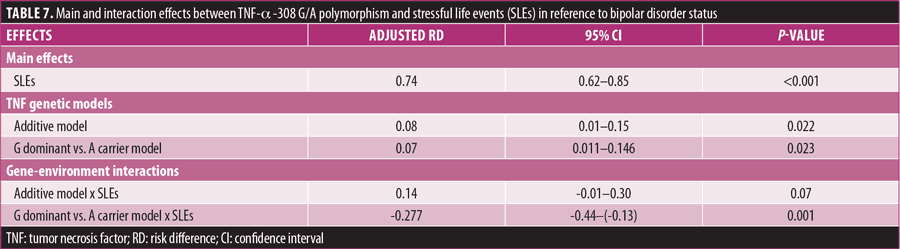
When interaction effects were analyzed, there was no significant interaction seen between the additive model (AA=0, GA=1, GG=2) and SLEs (RD: 0.14, 95% CI: -0.01–0.30, p=0.07). However, GG versus A carrier model (AA and GA=0, GG=1) revealed a strong significant interaction with SLEs (RD: -0.277, 95% CI: -0.44–[-0.13], p=0.001). It can be inferred that the interaction between SLEs and BD is significant and greater for those in the A carrier group, compared to those with GG genotype.
Substance abuse. Out of 400 patients with BD, 110 (27.5%) engaged in substance abuse, while 290 (72.5%) did not (Table 8). Most of the patients who engaged in substance abuse were male, and male sex was strongly related to substance abuse (p=0.001) in this population. In patients with substance abuse, age of onset of disease was significantly lower than those who did not engage in substance abuse (p=0.001). Family history of psychiatric illness was negatively associated with substance abuse (p=0.02). Most of the patients who engaged in substance abuse also reported smoking habits (p=0.001). When behavior of patients with substance abuse was analyzed, presence of psychotic symptoms, rapid recycling of mood, and disturbed cognitive behavior were significantly related to substance abuse, and there was no significant association between suicidal behavior and substance abuse (p=0.099). Taking into account socioeconomic status, people of low socioeconomic status were more often engaged in substance abuse, compared to those of middle or upper socioeconomic status (p=0.001). Analyzing the association between stages of BD and substance abuse, there was a strong association between manic/hypomanic condition and substance abuse (p=0.001).
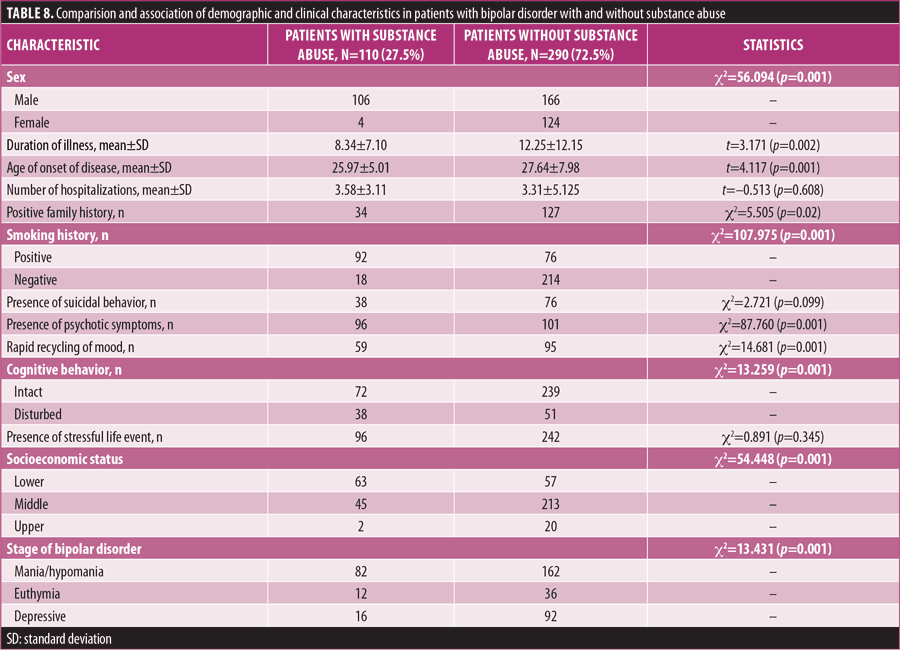
Logistic regression was applied to find an interaction between substance abuse and other genetic and environmental factors, with age and sex as controlling factors (Table 9). There was a significant association between socioeconomic status and substance abuse (OR: 0.077, p=0.046). Smoking history also remained strongly related to substance abuse (OR: 0.034, p=0.001). Logistic regression showed that suicidal behavior was significantly associated with substance abuse (p=0.011), while rapid recycling of mood and cognitive behavior lost their strong association. Presence of psychotic behavior remained significantly related to substance abuse (p≤0.001). Analysis of interaction between TNF-α -308G/A and substance abuse revealed a significant interactive relationship (β=1.031, OR: 2.804, p≤0.001).
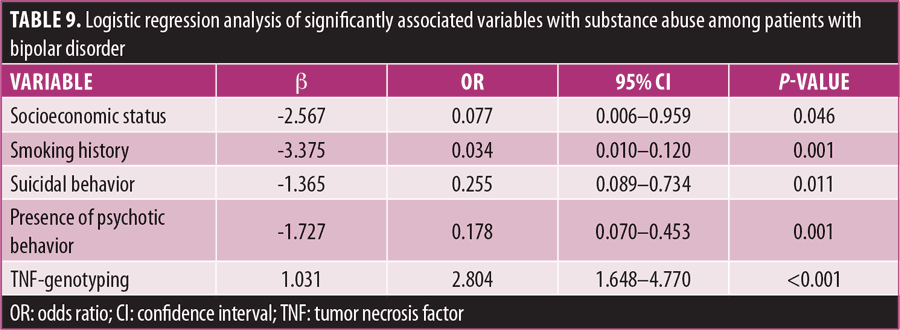
Type of substance used by patients with BD. The type and quantity of drugs used by subjects play a crucial role in their rehabilitation. Figure 3 shows types of substances used by subjects. Cannabis in different forms was the most used drug among patients with BD, followed by tobacco and tobacco snuff. Some of the patients used multiple substances in combination.
Bioinformatics analysis. STRING analysis of the TNF-α network showed that 51 proteins were interconnected through 438 connections (Figure 4). PPI enrichment p-value was less than 1.0×10-16, which showed that the observed number of connections was significant. Through analysis of KEGG pathway gene ontology (GO), the nodes were found to be significantly involved in many vital life processes.
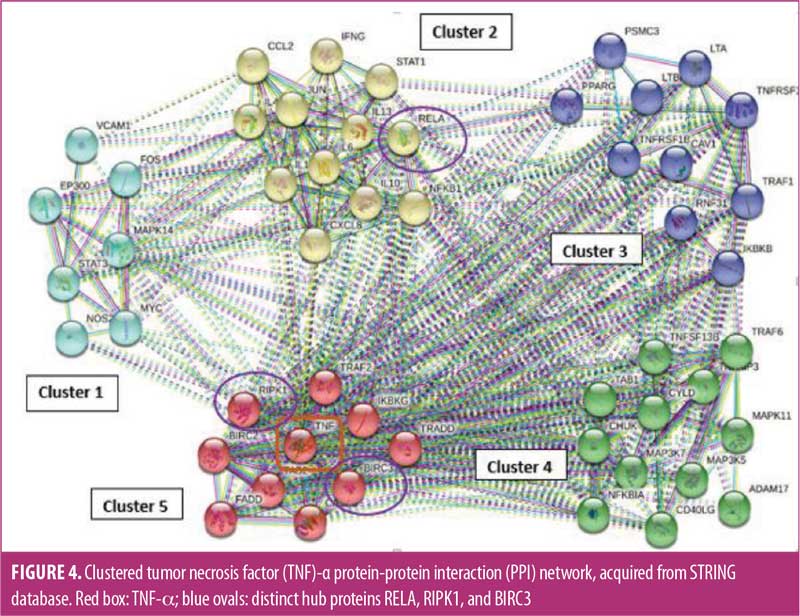
Analysis of clusters showed that cluster one comprised of seven proteins mainly acting through TNF, cAMP, and Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathways. The 11 proteins in cluster two were mostly chemotactic and transcription factors acting through chemokine, IL-17, Toll-like receptor, and nucleotide-binding oligomerization domain (NOD)-like receptor signaling mechanisms. Cluster three included TNF receptor superfamily members and protein folding regulatory proteins involved in cytokine-cytokine receptor interactions and nuclear factor kappa B (NF-κB) signaling mechanism. Cluster four was comprised of 12 proteins associated with the TNF family and mitogen-activated protein kinase family. Cluster five contained TNF and its associated proteins, along with proteins involved in apoptosis, mainly performing their action through mitogen-activated protein kinase (MAPK), TNF, IL-17, and retinoic acid-inducible gene I (RIG-1) signaling mechanism.
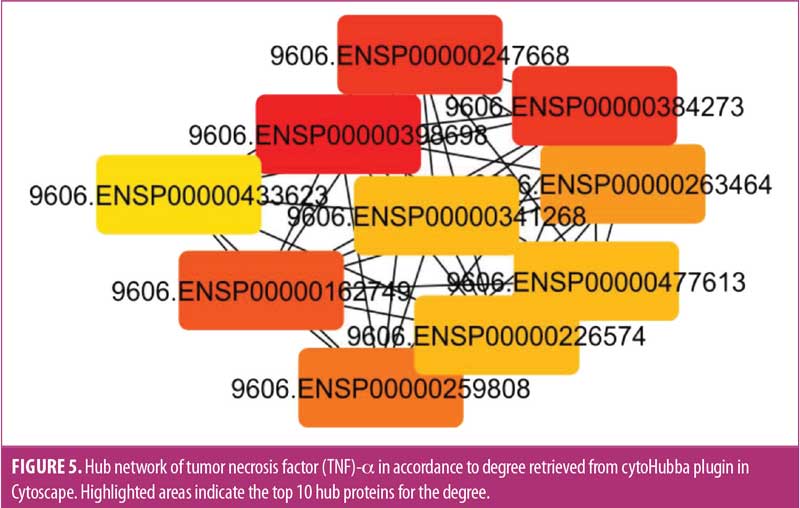
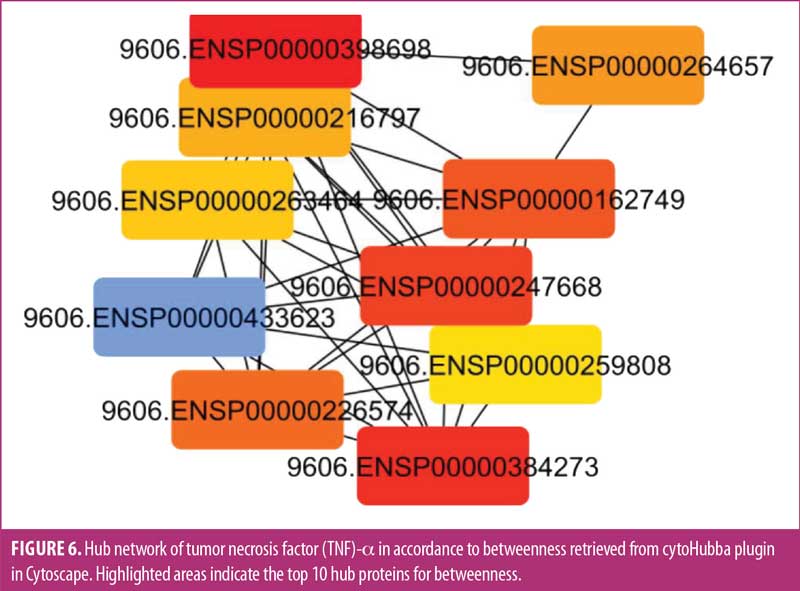
Hubness of the network was analyzed through cytoHubba for the degree (Figure 5) and betweenness (Figure 6) of the proteins. Among the top 10 proteins obtained by both methods, eight were common according to degree and betweenness. These included TNF receptor associated factor 6 (TRAF6), TNF, RELA, baculoviral IAP repeat-containing protein 3 (BIRC3), receptor-interacting serine/threonine-protein kinase 1 (RIPK1), TRAF2, NFκB1, and TNF receptor superfamily member 1A (TNFRSF1A). Of these eight proteins, TRAF6, TNF, TRAF2, NFκB1, and TNFRSF1A have been previously linked to BD.12,42–44 Their biological value and association with BD were further verified by the hubness of these proteins. Three distinct hub proteins having multiple interactions in the network, RELA, BIRC3, and RIPK1, have been identified (Figure 4). These three distinct genes can be employed as the latest targets for research, along with TNF and NFκB1, in patients with BD to understand the pathophysiology of the disease.
Discussion
BD is a complicated and multifactorial disease. Beside environmental and psychosocial factors, genetic alterations, specifically SNPs, might be associated with the pathogenesis of BD. We found a significant association between TNF-α -308G/A polymorphism and BD in our study, and this finding is in accordance with the previous studies in Korean,45 Polish,16 and Italian populations.46 Conversely, British,47 Brazilian,48 and Iranian49 studies reported no significant association between TNF-α -308G/A polymorphism and BD. These differences could be due to variations in environmental exposure and genetic makeup of different ethnic populations, and altered allelic frequencies in various ethnic populations might affect the outcome of the disease.50
We divided patients with BD into two groups according to positive family history of mood disorder and analyzed the clinical and functional features of the patients. We suggested that positive family history of mood disorder contributed to the adverse characteristics of BD. Rapid cycling of mood and tendency of suicidal behavior significantly correlated with positive family history. Increased tendency of suicidal behavior in persons with familial mood disorders has previously been reported.51 Berutti et al52 also studied the clinical characteristics of patients with BD comparing family history of psychiatric illness and found a strong association of suicidal tendency in patients with positive family history. It was inferred that cognitive ability did not significantly change in patients with positive familial history. This is contrary to McGrath et al,53 who found that familial psychiatric history slightly decreased the cognitive ability of patients. Our study also suggests that patients with BD and family history of psychiatric illness have increased rates of smoking and drug use. Our findings are in line with other research studies.52
SLEs significantly influence the onset, course, and recurrence of mood disorders.19 In patients with BD, exposure to stress might contribute to the first onset of a bipolar episode and progression of the disease.54 In our study, a significant association between exposure of some SLE and onset of BD was observed. Multiple researchers have conducted studies regarding stress and BD, and their findings support our results.55,56 Gershon et al19 reported that the severity of chronic stressors predicted the severity of depressive symptoms, but not manic symptoms, in patients with BD. Similarly, Kim et al54 found that stress was associated with mood changes in BD, and Sato et al20 determined that severity of depressive episodes was associated with the psychologically stressful event. Moreover, Kemner et al21 reported that life events not only increase the risk of the first admission in the hospital, but also affect recurrent admissions.
Considering the effect of stress on sex, it was determined that, although all patients experience negative life events at the onset of BD, experiencing negative life events was a stronger triggering factor for the onset of BD symptoms in female patients. The reason for this could be that male patients have a greater ability to cope with stress, as suggested by Mayor57 and Matud.58 Contrary to our findings, it has been reported that SLEs lead to depression in male individuals more strongly than in female individuals.59
Multiple environmental factors in association with genetic factors might play a role in the onset and progression of any disease. Since SLEs are potential risk factors for inflammation as well as BD, the association between SLEs before the onset of BD and TNF-α -308G/A polymorphism was analyzed. Interaction of SLEs with GG versus A carrier model demonstrated a significant association of the two with onset of the disease. Such G × E studies have been carried out between SLEs and other genes. Significant association of brain-derived neurotrophic factor (BDNF) gene polymorphism with SLEs was found by Hosang et al.36 Similarly, interaction of catechol-O-methyl-transferase (COMT) gene polymorphism with SLEs were analyzed with respect to worst manic and worst depressive episodes, and the significant impact of SLEs was depicted by the COMT genotype for the worst depressive episodes.60 Interactions between serotonin transporter gene, COMT gene, and SLEs in patients with mood disorders were established by Mandelli et al.61 Therefore, it is assumed that stressful events can be a triggering factor for the onset of BD, and any further stress in life can cause a relapse of either depressive or manic episodes.62
Many individuals involved in substance abuse are vulnerable to the development psychiatric illness and vice versa.63 Symptoms of patients with BD might be aggravated by substance use. There is the potential to misdiagnose BD as substance use disorder, due to the similarity in symptoms. Therefore, it is important to consider addiction as a risk factor for BD, and accurate diagnosis of psychiatric illness must be carried out for proper and early treatment.64 Moreover, by taking into account substance use along with psychiatric symptoms, effective treatments can be planned for each individual, and a better prognosis can be expected.
In this study, we found that substance abuse was associated with earlier onset of BD, which is in accordance with the results reported by Lagerberg et al,65 Strakowski et al,66 and Cardoso et al.31 Considering other risk factors associated with substance abuse, we found that male patients had a higher rate of substance abuse than female patients; this important finding has been previously reported in some investigations, suggesting that male patients are more vulnerable to substance abuse.67 Besides sex, socioeconomic status and income also play a critical role in the development of substance abuse. We found that individuals of low socioeconomic status were engaged in more substance abuse. Alnıak et al68 reported that a higher percentage of unemployed people used drugs. Charitonidi et al69 reported that individuals of higher socioeconomic status were involved in alcohol use, while people of low socioeconomic class used tobacco.
We found a significant association between substance abuse and smoking history. Most of the patients using drugs also had a smoking habit. Weinberger et al70 indicated that continued smoking or initiation of smoking can cause a relapse of drug use. While assessing the characteristics of BD, we also found a higher tendency of suicidal behavior in patients who used drugs. It has been reported that people engaged in substance use are more prone to suicidal thinking, whether they attempt suicide or not.71
A significant association was found between substance abuse and TNF-α -308G/A polymorphism. Previously, interactive and additive interaction of TNF genotype with substance use was analyzed, and a significant interaction between TNF -308G/A polymorphism and smoking, alcohol use, and quid chewing was observed.72
In our study, most of the patients with BD were found to use cannabis and tobacco in various forms. Maremmani et al30 reported that patients with BD were more susceptible to use cannabinoids, cocaine, amphetamines, and opiates than patients with schizophrenia.Alnıak et al68 reported that the most commonly used substances among patients with BD were cannabis and alcohol. The type of the drug used by the patient can affect the effectiveness of treatment. For patients with BD who engage in substance abuse, a more intensive therapeutic strategy must be considered to produce effective results.
The identification of new target proteins in BD can be helpful in understanding the biology of the disease; therefore, in-silico computational analysis of TNF-α protein with its interactors was conducted. Three distinct proteins, RELA, BIRC3, and RIPK1, were identified, which might have some significant association with BD. These three proteins are related to the NF-kB transcription factor, which regulates the expression of cytokines, immune and inflammatory systems, and apoptosis. RELA gene, also known as transcription factor p65, has been associated with the learning and memorizing ability of the brain, so it might be involved in altering the cognitive behavior of patients with BD.73 BIRC3 and RIPK1 are associated with apoptotic cascades and inflammatory pathways. They might be associated with neuroinflammation, which could lead to neural dysfunction, which might provide a link between these proteins and BD.74,75 These three proteins were identified by analyzing hub proteins in the network according to their betweenness and degree, in collaboration with TNF-α, and they might contribute as crucial proteins to the inflammatory mechanism related to BD. By analyzing the network, we depicted that there is an interaction between several pathways, such as TNF receptor, NF-kB, NOD-like receptor, Toll-like receptor, and multiple apoptotic pathways. These interactions could provide significant information that might help us understand the underlying mechanism of BD. Aside from their role in understanding the complexity of the disease, these proteins might act as therapeutic targets with improved response. Further investigations at a molecular level are required to confirm their involvement in the disease process.
Conclusion
This study suggests that TNF-α -308G/A polymorphism is significantly associated with BD in the Pakistani population. This association can further be affirmed with a larger sample size and different ethnic groups. Among multiple risk factors related to psychiatric illness, stress was found to be significantly associated with the onset of BD. Furthermore, substance abuse in male patients is also a critical risk factor of BD. Further investigations can focus on the prediction of new pathways and proteins as contributing factors and therapeutic targets for BD.
Acknowledgments
The authors acknowledge University of the Punjab in Lahore, Pakistan, for providing the funds and facilities for this research study.
References
- Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8(1):1.
- Rowland TA, Marwaha S. Epidemiology and risk factors for bipolar disorder. Ther Adv Psychopharmacol. 2018;8(9):251–269.
- Barbosa IG, Huguet RB, Mendonça VA, et al. Increased plasma levels of soluble TNF receptor I in patients with bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2011;261(2):139–143.
- McIntyre RS, Berk M, Brietzke E, Goldstein BI, et al. Bipolar disorders. Lancet. 2020;396(10265):1841–1856.
- Hu C-Y, Qian Z-Z, Gong F-F, et al. Methylenetetrahydrofolate reductase (MTHFR) polymorphism susceptibility to schizophrenia and bipolar disorder: an updated meta-analysis. J Neural Transm (Vienna). 2015;122(2):307–320.
- Benedetti F, Aggio V, Pratesi ML, et al. Neuroinflammation in bipolar depression. Front Psychiatry. 2020;11:71.
- Pereira AC, Oliveira J, Silva S, et al. Inflammation in bipolar disorder (BD): identification of new therapeutic targets. Pharmacol Res. 2021;163:105325.
- Muneer A. Bipolar disorder: role of inflammation and the development of disease biomarkers. Psychiatry Investig. 2016;13(1):18–33.
- Doğanavsargil Baysal Ö, Erdoğan A, Cinemre B, et al. Levels of TNF alpha, soluble TNF receptors (sTNF-R1, sTNF-R2) in bipolar disorder. Noro Psikiyatr Ars. 2019;57(2):136–140
- Barbosa IG, Vaz GN, Rocha NP, et al. Plasma levels of tumor necrosis factor superfamily molecules are increased in bipolar disorder. Clin Psychopharmacol Neurosci. 2017;15(3):269–275.
- Probert L. TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience. 2015;302:2–22.
- Barbosa IG, Bauer ME, Machado-Vieira R, Teixeira AL. Cytokines in bipolar disorder: paving the way for neuroprogression. Neural Plast. 2014;2014:360481.
- Ramírez-Bello J, Jiménez-Morales M. [Functional implications of single nucleotide polymorphisms (SNPs) in protein-coding and non-coding RNA genes in multifactorial diseases]. Gac Med Mex. 2017;153(2):238–250.
- Orrù G, Carta MG. Genetic variants involved in bipolar disorder, a rough road ahead. Clin Pract Epidemiol Ment Health. 2018;14:37–45.
- Sharma S, Ghosh B, Sharma SK. Association of TNF polymorphisms with sarcoidosis, its prognosis and tumour necrosis factor (TNF)-α levels in Asian Indians. Clin Exp Immunol. 2008;151(2):251–259.
- Czerski PM, Rybakowski F, Kapelski P, et al. Association of tumor necrosis factor –308G/A promoter polymorphism with schizophrenia and bipolar affective disorder in a Polish population. Neuropsychobiology. 2008;57(1–2):88–94.
- Kadasah S, Arfin M, Rizvi S, et al. Tumor necrosis factor-α; and –β genetic polymorphisms as a risk factor in Saudi patients with schizophrenia. Neuropsychiatr Dis Treat. 2017;13:1081–1088.
- Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984–1010.
- Kerner B. Genetics of bipolar disorder. Appl Clin Genet. 2014;7:33–42.
- Gershon A, Johnson SL, Miller I. Chronic stressors and trauma: prospective influences on the course of bipolar disorder. Psychol Med. 2013;43(12):2583–2592.
- Sato A, Hashimoto T, Kimura A, et al. Psychological distress symptoms associated with life events in patients with bipolar disorder: a cross-sectional study. Front Psychiatry. 2018;9:200.
- Kemner SM, van Haren NE, Bootsman F, et al. The influence of life events on first and recurrent admissions in bipolar disorder. Int J Bipolar Disord. 2015;3:6.
- Postal M, Lapa AT, Sinicato NA, et al. Depressive symptoms are associated with tumor necrosis factor alpha in systemic lupus erythematosus. J Neuroinflammation. 2016;13:5.
- Ma K, Zhang H, Baloch Z. Pathogenetic and therapeutic applications of tumor necrosis factor-α (TNF-α) in major depressive disorder: a systematic review. Int J Mol Sci. 2016;17(5):733.
- Lee EE, Hong S, Martin AS, Eet al. Inflammation in schizophrenia: cytokine levels and their relationships to demographic and clinical variables. Am J Geriatr Psychiatry. 2017;25(1):50–61.
- Luo Y, He H, Zhang J, et al. Changes in serum TNF-α, IL-18, and IL-6 concentrations in patients with chronic schizophrenia at admission and at discharge. Compr Psychiatry. 2019;90:82–87.
- Luo Y, He H, Zhang M, et al. Altered serum levels of TNF-α, IL-6 and IL-18 in manic, depressive, mixed state of bipolar disorder patients. Psychiatry Res. 2016;244:19–23.
- Saxena K, Okusaga O, Kahlon R, et al. F108. Plasma TNF-alpha is associated with stressful life events in youth with bipolar disorder. Biol Psychiatry. 2018;83(9 Suppl 1):S279.
- Messer T, Lammers G, Müller-Siecheneder F, et al. Substance abuse in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Res. 2017;253:338–350.
- Maremmani AGI, Bacciardi S, Gehring ND, et al. Substance use among homeless individuals with schizophrenia and bipolar disorder. J Nerv Ment Dis. 2017;205(3):173–177.
- Cardoso TA, Bauer IE, Jansen K, et al. Effect of alcohol and illicit substance use on verbal memory among individuals with bipolar disorder. Psychiatry Res. 2016;243:225–231.
- Brown GW, Harris TO, eds. Life Events and Illness. Guilford Press; 1989.
- Ormel J, Oldehinkel AJ, Brilman EI. The interplay and etiological continuity of neuroticism, difficulties, and life events in the etiology of major and subsyndromal, first and recurrent depressive episodes in later life. Am J Psychiatry. 2001;158(6):885–891.
- Gold AK, Otto MW, Deckersbach T, et al. Substance use comorbidity in bipolar disorder: a qualitative review of treatment strategies and outcomes. Am J Addict. 2018;27(3):188–201.
- Uher R, Caspi A, Houts R, et al. Serotonin transporter gene moderates childhood maltreatment’s effects on persistent but not single-episode depression: replications and implications for resolving inconsistent results. J Affect Disord. 2011;135(1–3):56–65.
- Hosang GM, Uher R, Keers R, et al. Stressful life events and the brain-derived neurotrophic factor gene in bipolar disorder. J Affect Disord. 2010;125(1–3):345–349.
- Szklarczyk D, Gable AL, Nastou KC, et al. The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49(D1):D605–D612.
- Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000 Jan 1;28(1):27–30.
- Su G, Morris JH, Demchak B, Bader GD. Biological network exploration with cytoscape 3. Curr Protoc Bioinforma. 2014;47:8.13.1–8.13.24.
- Chin C-H, Chen S-H, Wu H-H, et al. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl 4):S11.
- Barnett JH, Smoller JW. The genetics of bipolar disorder. Neuroscience. 2009;164(1):331–343.
- Hoseth EZ, Ueland T, Dieset I, et al. A study of TNF pathway activation in schizophrenia and bipolar disorder in plasma and brain tissue. Schizophr Bull. 2017;43(4):881–890.
- Zhou R, Wang F, Zhao G, et al. Effects of tumor necrosis factor-α polymorphism on the brain structural changes of the patients with major depressive disorder. Transl Psychiatry. 2018;8(1):217.
- Brietzke E, Kapczinski F. TNF-α as a molecular target in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1355–1361.
- Pae C-U, Lee K-U, Han H, et al. Tumor necrosis factor alpha gene-G308A polymorphism associated with bipolar I disorder in the Korean population. Psychiatry Res. 2004;125(1):65–68.
- Clerici M, Arosio B, Mundo E, et al. Cytokine polymorphisms in the pathophysiology of mood disorders. CNS Spectr. 2009;14(8):419–425.
- Middle F, Jones I, Robertson E, et al. Tumour necrosis factor alpha and bipolar affective puerperal psychosis. Psychiatr Genet. 2000;10(4):195–198.
- Meira-Lima IV, Pereira AC, Mota GF, et al. Analysis of a polymorphism in the promoter region of the tumor necrosis factor alpha gene in schizophrenia and bipolar disorder: further support for an association with schizophrenia. Mol Psychiatry. 2003;8(8):718–720.
- Ahrari S, Talaei A, Afshari J. No association of the TNF-α –G308A gene polymorphism with bipolar I disorder in a sample of Iranian population. 2016.
- Padyukov L, Hahn-Zoric M, Lau Y, Hanson L. Different allelic frequencies of several cytokine genes in Hong Kong Chinese and Swedish Caucasians. Genes Immun. 2001;2(5):280–283.
- Mann JJ, Bortinger J, Oquendo MA, et al. Family history of suicidal behavior and mood disorders in probands with mood disorders. Am J Psychiatry. 2005;162(9):1672–1679.
- Berutti M, Nery FG, Sato R, et al. Association between family history of mood disorders and clinical characteristics of bipolar disorder: results from the Brazilian bipolar research network. J Affect Disord. 2014;161:104–108.
- McGrath JJ, Wray NR, Pedersen CB, et al. The association between family history of mental disorders and general cognitive ability. Transl Psychiatry. 2014;4(7):e412.
- Kim EY, Miklowitz DJ, Biuckians A, Mullen K. Life stress and the course of early-onset bipolar disorder. J Affect Disord. 2007;99(1–3):37–44.
- Koenders M, Giltay E, Spijker A, et al. EPA-1204–Stressful life events in bipolar i and ii disorder: cause or consequence of mood symptoms? Eur Psychiatry. 2014;29(Suppl 1):1.
- Subramanian K, Sarkar S, Kattimani S, et al. Role of stressful life events and kindling in bipolar disorder: converging evidence from a mania-predominant illness course. Psychiatry Res. 2017;258:434–437.
- Mayor E. Gender roles and traits in stress and health. Front Psychol. 2015;6:779.
- Matud MP. Gender differences in stress and coping styles. Personal Individ Differ. 2004;37(7):1401–1415.
- Assari S, Lankarani MM. Stressful life events and risk of depression 25 years later: race and gender differences. Front Public Health. 2016;4:49.
- Hosang GM, Fisher HL, Cohen-Woods S, et al. Stressful life events and catechol-O-methyl-transferase (COMT) gene in bipolar disorder. Depress Anxiety. 2017;34(5):419–426.
- Mandelli L, Serretti A, Marino E, et al. Interaction between serotonin transporter gene, catechol-O-methyltransferase gene and stressful life events in mood disorders. Int J Neuropsychopharmacol. 2007;10(4):437–447.
- Sam SP, Nisha A, Varghese PJ. Stressful life events and relapse in bipolar affective disorder: a cross-sectional study from a tertiary care center of Southern India. Indian J Psychol Med. 2019;41(1):61–67.
- Ross S, Peselow E. Co-occurring psychotic and addictive disorders: neurobiology and diagnosis. Clin Neuropharmacol. 2012;35(5):235–243.
- Shalini Theodore R, Ramirez Basco M, Biggan JR. Diagnostic disagreements in bipolar disorder: the role of substance abuse comorbidities. Depress Res Treat. 2012;2012:435486.
- Lagerberg TV, Andreassen OA, Ringen PA, et al. Excessive substance use in bipolar disorder is associated with impaired functioning rather than clinical characteristics, a descriptive study. BMC Psychiatry. 2010;10:9.
- Strakowski SM, DelBello MP, Fleck DE, Arndt S. The impact of substance abuse on the course of bipolar disorder. Biol Psychiatry. 2000;48(6):477–485.
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12(12):559–566.
- Alnıak İ, Erkıran M, Mutlu E. Substance use is a risk factor for violent behavior in male patients with bipolar disorder. J Affect Disord. 2016;193:89–93.
- Charitonidi E, Studer J, Gaume J, et al. Socioeconomic status and substance use among Swiss young men: a population-based cross-sectional study. BMC Public Health. 2016;16:333.
- Weinberger AH, Platt J, Esan H, et al. Cigarette smoking is associated with increased risk of substance use disorder relapse: a nationally representative, prospective longitudinal investigation. J Clin Psychiatry. 2017;78(2):e152–e160.
- Esang M, Ahmed S. A closer look at substance use and suicide. Am J Psychiatry Resid J. 2018;13(6):6–8.
- Jeng J-E, Tsai H-R, Chuang L-Y, et al. Independent and additive interactive effects among tumor necrosis factor-α polymorphisms, substance use habits, and chronic hepatitis B and hepatitis C virus infection on risk for hepatocellular carcinoma. Medicine (Baltimore). 2009;88(6):349–357.
- Hashimoto R, Ohi K, Yasuda Y, et al. Variants of the RELA gene are associated with schizophrenia and their startle responses. Neuropsychopharmacology. 2011;36(9):1921–1931.
- Nazeen S, Palmer NP, Berger B, Kohane IS. Integrative analysis of genetic data sets reveals a shared innate immune component in autism spectrum disorder and its co-morbidities. Genome Biol. 2016;17(1):228.
- Mifflin L, Ofengeim D, Yuan J. Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target. Nat Rev Drug Discov. 2020;19(8):553–571.





