by Alok Singh, MD
Dr. Singh is with All India Institute of Medical Sciences in Raipur, India.
Funding: No funding was provided for this study.
Disclosures: The author has no conflicts of interest relevant to the contents of this article.
Innov Clin Neurosci. 2022;19(10–12):43–47.
Abstract
Xanomeline, a cholinergic agonist, was initially evaluated for the treatment of Alzheimer’s disease and schizophrenia. However, drug development was stopped due to the severe cholinergic adverse effects. In recent years, xanomeline has been explored, along with trospium, a peripheral cholinergic antagonist, for schizophrenia. Xanomeline acts primarily as an M1/M4 agonist and might lead to improvement in all symptom types of schizophrenia. Due to its role as an antimuscarinic agent, trospium is expected to reduce the adverse effects of xanomeline. In initial studies, this combination seems to be promising in the treatment of schizophrenia. The most common side effects of this combination included constipation, dry mouth, and nausea. This article summarizes the present status of combination xanomeline and trospium in schizophrenia.
Keywords: Schizophrenia, xanomeline, trospium
Schizophrenia is a disease of diverse etiology, involving numerous neurotransmitters (e.g., dopamine, 5-hydroxytryptamine [5-HT], and acetylcholine), which results in a variety of symptoms.1 It is characterized by positive and negative symptoms and cognitive impairment. The positive symptoms exaggerate standard processes, leading to hallucinations, delusions, and disorganized thoughts and/or behavior. Conversely, negative symptoms are characterized by diminution of physiologic processes (e.g., flat affect, apathy, and anhedonia). Cognitive impairments include alterations in memory, learning, and attention.2 Traditionally, positive symptoms have been attributed to dopaminergic hyperactivity in mesolimbic pathways, and negative and cognitive symptoms have been attributed to hypodopaminergic activity in mesocortical pathways.1,3 It has also been suggested that negative symptoms are caused by the perturbations of neurotransmission involving numerous neural networks in the brain, such as the frontocortico temporal networks and corticostriatal loops.4 The diffuse involvement of networks might also contribute to reduced responsiveness of negative symptoms to current drug therapies. Positive symptoms are most amenable to drug therapy, whereas negative symptoms and cognitive impairments are difficult to treat.5,6 Currently, there is no specific drug approved by the United States (US) Food and Drug Administration (FDA) to treat the negative symptoms of schizophrenia.3
The pharmacotherapy of schizophrenia primarily relies on drugs modulating the activity of dopamine and 5-HT. Broadly, two different generations of drugs are used to manage schizophrenia, characterized by ratio of affinity toward D2/5-HT2A. First-generation drugs have a high D2/5-HT2A ratio, whereas newer ones have a low D2/5-HT2A ratio.7 Older drugs are readily available and inexpensive, with significant antimuscarinic (e.g., dry mouth, constipation, difficulty urinating), anti-adrenergic (e.g., orthostatic hypotension, impotence), endocrine (e.g., amenorrhea, galactorrhea, impotence), and central nervous system (CNS) (e.g., Parkinson’s disease, akathisia, tardive dyskinesia, acute dystonic reactions) adverse effects. Weight gain and metabolic abnormalities (e.g., hyperglycemia, insulin resistance, hyperlipidemia) can also occur with long-term usage and are more common with atypical antipsychotic agents, such as clozapine and olanzapine.8 The criteria of minimal response (≥20% Positive and Negative Syndrome Scale [PANSS]/Brief Psychiatric Rating Scale [BPRS] score reduction) is achieved by more patients with first-episode schizophrenia, compared to patients with multiple episodes of schizophrenia (81% vs. 51%); however, the criteria for good response (≥50% PANSS/BPRS score reduction) is fulfilled by a relatively lower number of both first- and multiple-episode patients (52% vs. 23%).9 Current drugs have shown significant efficacy, compared to placebo, in the prevention of relapse after 7 to 12 months of illness (risk ratio: 0.38 [0.32, 0.45]; p<0.00001). These results must be analyzed carefully, as the studies included were highly heterogeneous (I2=71%).10 It is evident that, although current drugs effectively manage the first episode of schizophrenia, only half of patients show an excellent response to these drugs, and drugs are effective in preventing relapse of schizophrenia. Considering the limitations of current drugs in terms of efficacy and adverse effects, new drugs that are safer and more effective are needed in the treatment of schizophrenia, especially for negative and cognitive symptoms.
Clinical Pharmacology
Xanomeline is a muscarinic agonist (M1 and M4) that was initially evaluated for the treatment of Alzheimer’s disease, followed by schizophrenia. In the CNS limbic and cortical regions have M1, M4, and dopaminergic receptors, which control cognition and affect.11 Animal studies have shown the role of M4 and M1 receptors in modulating psychotic and behavioral disturbances and correction of negative and/or cognitive symptoms, respectively.12,13 This explains the possible mechanism of action of xanomeline in schizophrenia. The time to reach maximum plasma concentration (Tmax) for xanomeline is 2.5 hours, and maximum plasma concentration following xanomeline 150mg is 13.8ng/mL.14 Xanomeline has very poor bioavailability (<1%) due to the extensive first-pass metabolism.15 Xanomeline is widely distributed, including to the CNS in animal studies, and the majority of the drug is excreted via the kidneys within 24 hours of administration.14
Clinical Studies
Xanomeline (oral and transdermal) for Alzheimer’s disease was discontinued in 1998 due to adverse effects.16,17 Similarly, the development of xanomeline for schizophrenia was abandoned, despite a favorable efficacy profile, due to the excessive peripheral cholinergic adverse effects (e.g., diarrhea, sweating, hypersalivation).18 Evaluation of xanomeline in the treatment of schizophrenia regained momentum when combined with trospium, a quaternary molecule that is a nonselective antimuscarinic agent devoid of CNS effects.19 The bioavailability of xanomeline, when administered with trospium, was similar in healthy volunteers, compared to patients with schizophrenia. Furthermore, when administered with tropsium, xanomeline achieved 10 percent more plasma concentration, compared to xanomeline alone.20
Simultaneous administration of both drugs should alleviate the cholinergic adverse effects. This hypothesis was substantiated in a Phase I trial.21,22 This randomized, double-blind, clinical trial aimed to assess the tolerability of the combination of xanomeline and trospium, compared to xanomeline and placebo. Before starting the trial, participants (n=70) received either placebo or trospium chloride (20mg twice daily) for two days, followed by xanomeline 75mg three times daily (total: 225mg) and trospium 20mg twice daily (total: 40mg) or xanomeline 75mg three times daily (total: 225mg) and placebo for seven days. The primary endpoint was designed to assess cholinergic adverse events as a mean weekly maximum composite Visual Analogue Scale (VAS) score (i.e., nausea, diarrhea, sweating, salivation, and vomiting combined). The reduction in VAS composite score was not statistically significant (p=0.31). The combination of xanomeline and trospium was shown to have significantly less cholinergic adverse effects (p=0.016), compared to xanomeline and placebo (34.3% vs. 63.6%).22 Individual cholinergic adverse effects were nausea (17.1% vs. 24.2%), vomiting (5.7% vs. 15.2%), diarrhea (5.7% vs. 21.2%), excess sweating (20.0% vs. 48.5%), and excess salivation (25.7% vs. 36.4%) for xanomeline and trospium and xanomeline and placebo, respectively. The trial showed an acceptable tolerability profile of xanomeline and trospium.
In another Phase I randomized, double-blind, placebo-controlled, multiple ascending-dose, clinical trial, the tolerability of different dose combinations of xanomeline and trospium was assessed in 69 healthy volunteers.23,24 The fixed ratio combinations of xanomeline and trospium were 100/20mg, 125/40mg, 150/20mg, and 150/40mg, administered twice daily. On Days 1 and 2, xanomeline (50mg) and trospium (20mg) were given twice daily, followed by different doses according to patient groups for next five days. The primary endpoint was the incidence of cholinergic adverse events of nausea, vomiting, diarrhea, excessive sweating, and salivation.The incidence of cholinergic adverse vents was 33 and 39 percent in 100mg and 125mg groups, respectively. Therefore, the combinations of xanomeline and trospium 100/20mg and 125/40mg were well tolerated. Most cholinergic adverse effects encountered were mild and lasted for less than three hours during the trial period.23,24 Further details of results from the 150/20mg and 150/40mg groups are still needed.
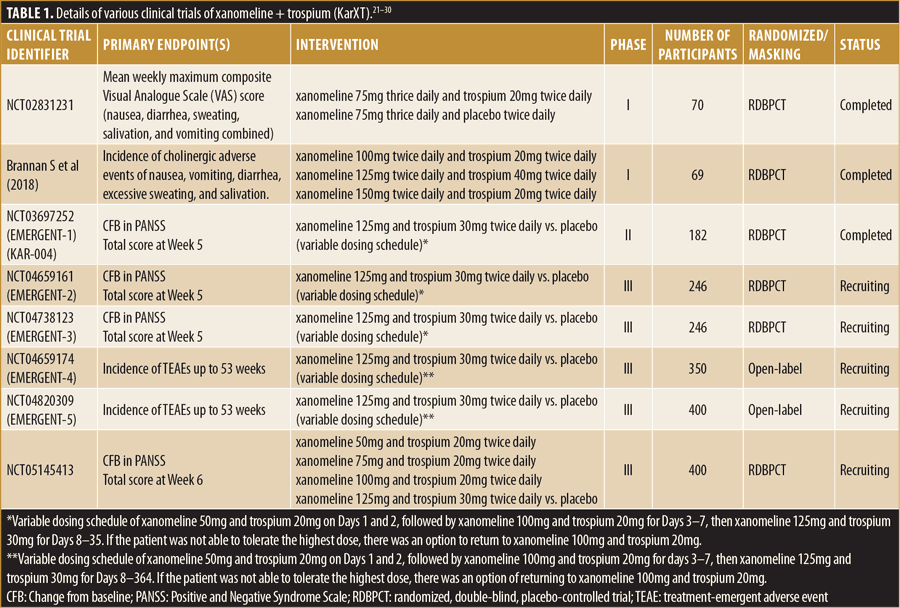
In a recently published Phase II randomized, double-blind, placebo-controlled clinical trial, the combination xanomeline and trospium (KarXT) was evaluated in acute exacerbations of schizophrenia.25 The trial involved patients aged 18 to 60 years diagnosed with schizophrenia, as per the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). Participants with resistant schizophrenia were excluded from the trial. The dose was titrated from twice daily xanomeline 50mg and trospium 20mg (Days 1 and 2), followed by xanomeline 100mg and trospium 20mg from Days 3 to 7, to a maximum of xanomeline 125mg and trospium 30mg twice daily (Days 8–34). The participants could receive the 100mg and 20mg twice daily doses if they were unable to tolerate the highest dose. Participants received 200 to 250mg/day and 40 to 60 mg/day of xanomeline and trospium, respectively. The primary endpoint was the change from baseline (CFB) to Week 5 in PANSS total score. Other essential endpoints were changes in PANSS positive and negative symptom subscore. Safety was assessed by measuring clinical lab parameters, weight, electrocardiography changes, suicidal tendency, and extrapyramidal symptoms. CFB in total PANSS score was -17.4 and -5.9 for xanomeline and trospium and placebo, respectively (p<0.001). For positive and negative symptoms, CFB was -5.6 and -2.4 (p<0.001) and -3.2 and -0.9 (p<0.001), respectively. Combination xanomeline and trospium significantly improved positive and negative symptoms; however, positive symptoms improved more than negative symptoms. The incidence of any adverse event was higher in the xanomeline and trospium group than placebo group (54% vs. 43%). Major adverse events were constipation (17%), nausea (17%), dry mouth (9%), dyspepsia (9%), and vomiting (9%) in the xanomeline and trospium group. Both groups’ mean weight gain (kg) was comparable (1.5kg vs. 1.1kg). The incidence of extrapyramidal symptoms was similar in both groups. Tachycardia was more prevalent in the xanomeline and trospium group. However, no QTc prolongation was noted in any participants. Combination xanomeline and trospium also resulted in both cholinergic and anticholinergic adverse effects.25 Currently, this is the only published trial regarding the efficacy and safety of combination xanomeline and trospium. Numerous clinical trials (NCT04659161, NCT04659174, NCT04738123, NCT04820309, and NCT05145413) are ongoing to assess the efficacy and safety of xanomeline and trospium and are included in Table 1.26–30
Discussion
The rationale of combining trospium and xanomeline was to counteract the troublesome cholinergic adverse effects of xanomeline, which previously led to its discontinuation, and both drugs are likely to be available as a fixed dose combination (FDC). The expected advantages of the FDC will be reduced pills, a simple treatment plan, and fewer chances of medication error; however, the major disadvantage will be difficulty in altering the dose of either drug without changing the dose of another. The flexible dosing regimen was applied in a clinical trial, but it might be difficult to implement in a real-world setting, whether prescribed individually or as an FDC. The mixture of cholinergic and anticholinergic adverse effects will further complicate the dose modification. A relatively higher incidence of anticholinergic adverse events was observed in the trial, which is contradictory to the cholinergic adverse events when xanomeline was used alone. This could be due to excess activity of trospium compared to xanomeline, which might be attributable to a discrepancy in the half-life of the drugs, though xanomeline dissociates very slowly from the receptors (trospium: T1/2: 20h vs. xanomeline: 1.25h).31,32 The half-life will be a significant factor in the preparation of an FDC. This could be one of the few unique FDCs where a drug is combined to reduce the adverse effects of one drug, as typically, FDCs lead to increased efficacy. The refinement of the dose-response relationship is required to predict and reduce the adverse effects. The disadvantage of this combination will be that without trospium, xanomeline cannot be prescribed.
The anticholinergic adverse effects encountered during the trial (e.g., constipation, dry mouth, somnolence) might not be well tolerated in some patient populations, especially in geriatric patients, decreasing the overall acceptability of the combination drug.25 The combination should be avoided in patients with a history of urinary retention, gastric retention, and narrow-angle glaucoma. Trospium is recommended at the lowest dose (20mg once daily) in patients with severe renal impairment (creatinine clearance <30mL/min) and patients over the age of 75 years.33 Hence, both drugs should be prescribed at the minimum doses in such patients.
Another important factor is the oral bioavailability of xanomeline, which is less than one percent. In animal studies, xanomeline has been shown to reach the CNS more than plasma, possibly explaining the CNS effects.14 Moreover, trospium is combined with xanomeline as a peripheral anticholinergic agent, but trospium itself has central anticholinergic effects, including somnolence, dizziness, hallucinations, and confusion.33 Thus, trospium can potentially antagonize the effects of xanomeline in the CNS.
Conclusion
Xanomeline is a cholinergic drug and is likely to avoid causing the significant adverse effects of existing drugs for schizophrenia. Around one-third of patients who did not respond to existing drugs might benefit from xanomeline since it acts through a different mechanism.34 Negative and cognitive symptoms of schizophrenia respond less to existing drugs; xanomeline provides an alternative new option in the management of schizophrenia, as it is shown to improve both positive and negative symptoms. If a more precise titration of doses of xanomeline and trospium can be achieved and adverse effects reduced, the combination could be instrumental in the treatment of schizophrenia, as it is efficacious in treating almost all the symptoms of schizophrenia.
References
- Singh A. Implications of istradefylline induced hallucinations. Encephale. 2021;47(2):179–180.
- Hany M, Rehman B, Azhar Y, Chapman J. Schizophrenia. 2021. In: StatPearls. StatPearls Publishing; 2022.
- Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–534.
- Millan MJ, Fone K, Steckler T, Horan WP. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. 2014;24(5):645–692.
- Blackman RK, Dickinson D, Eisenberg DP, et al. Antipsychotic medication-mediated cognitive change in schizophrenia and polygenic score for cognitive ability. Schizophr Res Cogn. 2021;27:100223.
- Aleman A, Lincoln TM, Bruggeman R, et al. Treatment of negative symptoms: where do we stand, and where do we go? Schizophr Res. 2017;186:55–62.
- DeBattista C. Antipsychotic agents and lithium. Katzung BG, ed. In: Basic and Clinical Pharmacology, 14th edition. McGraw-Hill Education; 2018:511–531.
- Meyer JM. Pharmacotherapy of Psychosis and Mania. Brunton LL, Hilal-Dandan R, Knollmann BC, eds. In: The Pharmacological Basis of Therapeutics, 13th edition. McGraw-Hill Education; 2018: 279–302.
- Haddad PM, Correll CU. The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther Adv Psychopharmacol. 2018;8(11):303–318.
- Ceraso A, Lin JJ, Schneider-Thoma J, et al. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2020;8:CD008016.
- Walker LC, Lawrence AJ. Allosteric modulation of muscarinic receptors in alcohol and substance use disorders. Adv Pharmacol. 2020;88:233–275.
- Foster DJ, Bryant ZK, Conn PJ. Targeting muscarinic receptors to treat schizophrenia. Behav Brain Res. 2021;405:113201.
- Moran SP, Maksymetz J, Conn PJ. Targeting muscarinic acetylcholine receptors for the treatment of psychiatric and neurological disorders. Trends Pharmacol Sci. 2019;40(12):1006–1020.
- Mirza NR, Peters D, Sparks RG. Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists. CNS Drug Rev. 2003;9(2):159–186.
- Davie BJ, Christopoulos A, Scammells PJ. Development of M1 mAChR allosteric and bitopic ligands: prospective therapeutics for the treatment of cognitive deficits. ACS Chem Neurosci. 2013;4(7):1026–1048.
- Adis Insight. Xanomeline. Updated 3 Jul 2019. https://adisinsight.springer.com/drugs/800001727. Accessed 18 Jan 2022.
- Bakker C, Prins S, Liptrot J, et al. Safety, pharmacokinetics and pharmacodynamics of HTL0009936, a selective muscarinic M1 -acetylcholine receptor agonist: a randomized cross-over trial. Br J Clin Pharmacol. 2021;87(11):4439–4449.
- Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165(8):1033–1039.
- Miller A, Kavoussi R, Breier A. Xanomeline plus tropsium: a novel strategy to enhance pro-muscarinic efficacy and mitigate peripheral side effects. Neuropsychopharmacology. 2016;41:S230.
- Karuna Therapeutics. Karuna Therapeutics announces results from Phase 1b trial evaluating the safety and tolerability of KarXT in healthy elderly volunteers. 23 Jun 2021. https://investors.karunatx.com/news-releases/news-release-details/karuna-therapeutics-announces-results-phase-1b-trial-evaluating/. Accessed 2 Feb 2022
- ClinicalTrials.gov. Pilot study comparing effects of xanomeline alone to xanomeline plus trospium. https://clinicaltrials.gov/ct2/show/NCT02831231. Accessed 18 Jan 2022.
- Kavoussi R , Miller A, Breier A. Results of a double-blind, placebo-controlled, tolerability study of KarXT: a novel combination targeting muscarinic acetylcholine receptors using xanomeline with trospium chloride to mitigate cholinergic side effects. Poster presented at: American Society of Clinical Psychopharmacology Annual Meeting; May 29–June 2, 2017; Miami Beach, FL.
- Brannan S, Miller A, Felder C, et al. KARXT: a M1/M4 preferring muscarinic agonist for the treatment of schizophrenia. Schizophr Bull. 2019; 45(Suppl 2): S244–S245.
- Brannan S, Miller A, Paul S, Breier A. KarXT, a combination of the M1/M4 cholinergic receptor agonist xanomeline and trospium for the treatment of psychosis and cognitive impairment in schizophrenia: Phase I studies. ACNP 57th Annual Meeting: Poster Session I. Neuropsychopharmacol. 2018;43(Suppl 1): 77–227.
- Brannan SK, Sawchak S, Miller AC, et al. Muscarinic cholinergic receptor agonist and peripheral antagonist for schizophrenia. N Engl J Med. 2021;384(8):717–726.
- ClinicalTrials.gov. A study to assess efficacy and safety of KarXT in acutely psychotic hospitalized adult patients with schizophrenia (EMERGENT-2). https://clinicaltrials.gov/ct2/show/NCT04659161. Accessed 2 Feb 2022.
- ClinicalTrials.gov. A study to assess efficacy and safety of KarXT in acutely psychotic hospitalized adult patients with schizophrenia (EMERGENT-3). https://clinicaltrials.gov/ct2/show/NCT04738123. Accessed 2 Feb 2022.
- ClinicalTrials.gov. An extension study to assess long-term safety, tolerability, and efficacy of KarXT in adult patients with schizophrenia (EMERGENT-4). https://clinicaltrials.gov/ct2/show/NCT04659174. Accessed 2 Feb 2022.
- ClinicalTrials.gov. An open-label study to assess the long-term safety, tolerability, and efficacy of KarXT in adult patients with schizophrenia (EMERGENT-5). https://clinicaltrials.gov/ct2/show/NCT04820309. Accessed 2 Feb 2022.
- ClinicalTrials.gov. A study to assess efficacy and safety of adjunctive KarXT in subjects with inadequately controlled symptoms of schizophrenia (ARISE). https://clinicaltrials.gov/ct2/show/NCT05145413. Accessed 2 Feb 2022.
- Bender AM, Jones CK, Lindsley CW. Classics in chemical neuroscience: xanomeline. ACS Chem Neurosci. 2017;8(3):435–443.
- Jakubík J, Tucek S, El-Fakahany EE. Allosteric modulation by persistent binding of xanomeline of the interaction of competitive ligands with the M1 muscarinic acetylcholine receptor. J Pharmacol Exp Ther. 2002;301(3):1033–1041.
- United States Food and Drug Administration. Sanctura (tropsium chloride) tablets label. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021595s009lbl.pdf. Accessed 19 Jan 2021.
- Nucifora FC Jr, Woznica E, Lee BJ, et al. Treatment resistant schizophrenia: clinical, biological, and therapeutic perspectives. Neurobiol Dis. 2019;131:104257.


