
by Andrew Yimu Vassantachart, MD; Elizabeth Yeo, MD; and Brian Chau, MD
Dr. Vassantachart is with Loma Linda University School of Medicine in Loma Linda, California. Dr. Yeo is with the Department of Physical Medicine and Rehabilitation, Loma Linda University Health, in Loma Linda, California. Dr. Chau is with the Department of Physical Medicine and Rehabilitation, Loma Linda University Health, in Loma Linda, California, and the Department of Physical Medicine and Rehabilitation, United States Department of Veterans Affairs in Loma Linda, California.
Funding: No funding was provided for this study.
Disclosures: The authors have no conflicts of interest relevant to the contents of this article.
Innov Clin Neurosci. 2022;19(10–12):48–57.
Abstract
Objective: To evaluate the literature on the effectiveness of virtual reality (VR)- and augmented reality (AR)-based treatments for phantom limb pain (PLP) in postamputation or brachial plexus avulsion (BPA) populations.
Methods: Multiple databases were queried in July 2021 with the keywords “virtual reality,” “augmented reality,” and “phantom limb pain.” Included studies utilized VR or AR to treat PLP with outcome measurement. Two independent reviewers assessed methodological quality using the Physiotherapy Evidence Databsae (PEDro) Scale and the Methodological Index for Nonrandomized Studies (MINORS) scoring. Studies were separated into immersive and nonimmersive AR/VR systems, with further categorization according to the specific methodologies used.
Results: Of 110 results from the database queries, 20 publications met the inclusion criteria. There was one unblinded, randomized, control trial (RCT), one single-blinded, randomized, crossover trial (RCxT), three comparative case series, 13 noncomparative case series, and two case reports. Seven of the 20 studies were classified as nonimmersive. Six studies reported decreased PLP after AR/VR treatments, of which four reported significant reductions. One study reported a reduction in PLP with no significant difference from control conditions. Thirteen of the 20 studies were classified as immersive AR/VR. Twelve studies reported decreased PLP after AR/VR treatments, of which eight reported significant reductions. One study found no change in PLP, compared to baseline.
Conclusions: The number of studies using AR/VR in PLP treatment has expanded since a 2017 review on the topic. The majority of these studies offer support for the efficacy of treating PLP with AR/VR-based treatments. Research has expanded on the customization, outcome measurements, and statistical analysis of AR/VR treatments. While results are promising, most publications remain at the case series level, and clinical indications should be cautioned. With improvements in the quality of evidence, there remain avenues for further investigations, including increased sampling, randomization, optimization of treatment duration, and comparisons to alternative therapies.
Keywords: Virtual reality (VR), augmented reality (AR), phantom limb pain, brachial plexus avulsion, amputation
Every year in the United States (US), approximately 185,000 persons undergo a limb amputation. Aside from challenges a patient might experience adapting to the anatomical loss, limb amputations can be complicated by a variety of post-procedure pains and sensations, including phantom limb pain (PLP).1 For individuals with limb amputations, the lifetime prevalence of PLP is estimated to be between 50 to 80 percent.2 PLP should be separated from pains localized to the amputated stump and is often described as burning, gnawing, stabbing, pressure, or projected pain that extends into the region previously occupied by the lost limb.1,3 PLP duration can vary from acute to chronic and can cause restrictions in postamputation activity and mobility, leading to adverse effects on quality-of-life.4
Despite a long history of documentation, there continues to be debate behind the actual mechanism of PLP, with the most popular theory being the cortical remapping theory (CRT).5,6 The CRT posits that postamputation, surrounding cortical somatosensory regions expand and invade the somatosensory regions originally mapped to the amputated limb, causing altered sensations and PLP.5,6
Currently, there is a variety of treatment options for PLP, including electrical stimulation, classical mirror therapy (MT), transcutaneous electrical nerve stimulation (TENS), and pharmacological treatments, such as gabapentin, morphine, ketamine, and dextromethorphan. However, while some studies have shown that these therapeutic choices have benefits over placebo, current guidelines for treating PLP remain unclear.7,8
MT was first implemented in the early 1990s for the treatment of PLP.9 MT consists of reflecting a patient’s intact limb with physical mirrors to provide the visual stimulus of having a complete limb set. There are several proposed mechanisms for why MT reduces PLP. One theory suggests that visualization of the mirrored limb helps the amputee gain a sense of control over the phantom limb, thereby reducing perceived sensory input and pain. Another theory suggests that visualizing the mirrored limb provides corrective visual inputs to help balance the discrepancy between motor outputs and sensory feedback.9 However, in a 2016 systematic review on the use of MT for PLP, there was not sufficient high-level evidence to support MT efficacy, due to variability in MT implementation and lack of a consensus on optimum frequency and duration of MT sessions.1 Several researchers have proposed that the use of physical mirrors limits the level of realism and immersion, which could contribute to the mixed effectiveness of MT.10 Recent advances in commercially available virtual reality (VR) and augmented reality (AR) systems might offer advantageous immersion and customizability, potentially overcoming proposed weaknesses in MT. VR systems create an entire scene in which both the subjects and the environment are computer generated images, while AR systems often utilize computer generated subjects superimposed on a real-life environment.11 VR and AR offer the opportunity to create a virtual limb that an individual with an amputated limb can view in three-dimensional space and use to interact with the environment. Additionally, the ability to create virtual environments allows for the ability to tailor to patient interests, encouraging maximum engagement and compliance in therapy.12
With increasing developments and research toward the integration of AR/VR augmented therapies for PLP, it is important to monitor the progression of this novel treatment to evaluate its effectiveness and determine its strengths and weaknesses. In 2017, Dunn et al13 performed a literature review encompassing studies that utilized VR for the treatment of PLP published up to that date. They concluded that although studies supported the use of VR therapies for the treatment of PLP, studies were limited by low levels of evidence, a lack of evaluation of long-term benefits, and inconsistency between stduy protocols.13 With newer studies continuously being published since the 2017 literature review, our aim was to perform an up-to-date systematic review of all investigations of AR/VR therapies in the treatment of PLP to reevaluate their use as a viable treatment modality.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used in the design of this study.14
Search strategy/information sources. The studies gathered in the 2017 literature review by Dunn et al13 were included in this systematic review. To collect newer studies, in July 2021, multiple databases (Medline/Pubmed, EMBASE, Physiotherapy Evidence Database [PEDro], and Cochrane) were searched for articles published from 2017 to July 2021 using the keywords “virtual reality” and “augmented reality,” with “phantom limb pain.” After duplicate articles were removed, abstracts of all articles were read in full, and those indicating use of VR or AR for the treatment of PLP were critiqued for matching inclusion criteria.
Eligibility criteria/selection process. To be included, studies needed to 1) be published in English, 2) include participants with PLP either from amputation or neurologic injury, 3) utilize any form of VR or AR therapy, and 4) assess the treatment of PLP using VR or AR therapy. Aside from the 2017 literature review by Dunn et al, articles that represented prior literature reviews or did not represent a research study were excluded from this review.
Data extraction. Data was extracted separately by two authors (AV, EY), who used a table specifically designed for this review. Extracted data included 1) study author name and publication date, 2) design of the study, 3) data for PEDro Scale and Methodological Index for Nonrandomized Studies (MINORS) scoring for respective studies, 4) sample size, 5) mean age of study participants, 6) description of intervention, 7) outcome measures, and 8) primary findings of the study. Any discrepancies between analysis were resolved by discussion among all authors for consensus.
Studies were divided into immersive and nonimmersive AR/VR environments. To be classified as an immersive AR/VR study, participants had to use some form of a head mounted display (HMD) that displayed virtual objects in a completely VR or AR environment. Due to the diverse array of VR and AR systems utilized by the immersive and nonimmersive studies, studies were then further divided by the modality of VR or AR utilized.
Methodological quality. The quality of the included studies was assessed by reviewers (AV, EY) who used the PEDro Scale for randomized, controlled trials (RCTs) and randomized crossover trials (RCxT) and MINORS scoring for non-RCT studies. The PEDro Scale is a validated tool with an 11-point scale used to evaluate the methodological quality of RCTs, with scores of 0 to 3 considered “poor,” 4 to 5 “fair,” 6 to 8 “good,” and 9 to 10 “excellent.”15,16 MINORS is a validated instrument used to assess the methodological quality of non-RCTs, with a maximum score of 16 for noncomparative studies and 24 for comparative studies.17
Results
Study selection. The eight articles in the 2017 literature review by Dunn et al13 were included in this review. The initial searches from the multiple databases between 2017 and July 2021 yielded a total of 110 articles. After the removal of duplicate studies in the various databases, 58 articles remained, and their abstracts were reviewed in full. Twelve of the 58 articles were found to meet the inclusion criteria, with common causes of exclusion being a lack of AR/VR intervention or unrelatedness to the treatment of PLP. In total, 20 studies were included in this systematic review, eight studies from the 2017 literature review and 12 that fit inclusion criteria from 2017 until July 2021.
Risk of bias in studies. Study quality and level of evidence varied across the included studies. One study was an unblinded RCT, with a PEDro Scale score of 8, and another study was a single-blinded RCxT, with a PEDro Scale score of 8. Three studies were comparative case series, with an average MINORS score of 17.3. Thirteen studies were noncomparative case series, with an average MINORS score of 10.8, and two studies were case reports with an average MINORS score of 8.18–36
Study protocols. Protocols of studies varied in number and duration of interventions and use of a follow-up evaluation. For studies that reported specific protocols, participants underwent an average of 9.2 interventions, with an average duration of 35.3 minutes. Out of the 20 included studies, seven utilized a follow-up period, which ranged from eight days to one year.
Outcome measures used. Various assessments for characteristics and measures of PLP were used. The most common outcome measure used was the short-form McGill Pain Questionnaire (SF-MPQ); 15 of the included studies utilizing this measure.
Nonimmersive studies. Out of the 20 included studies, seven were classified as nonimmersive. Study types included RCT (1), RCxT (1), case series (4), and case study (1). The average PEDro Scale score for the RCT and RCxT was 8, and average MINORS scoring for the non-RCTs was 11.2.18–24 Various VR implementations were used, including creation of a virtual limb from a mirror image of an intact limb, control of a virtual limb via electromagnetic sensors, creation of a brain-computer interface, and use of a tablet-based virtual treatment. General characteristics of the nonimmersive studies were extracted and synthesized in Table 1.
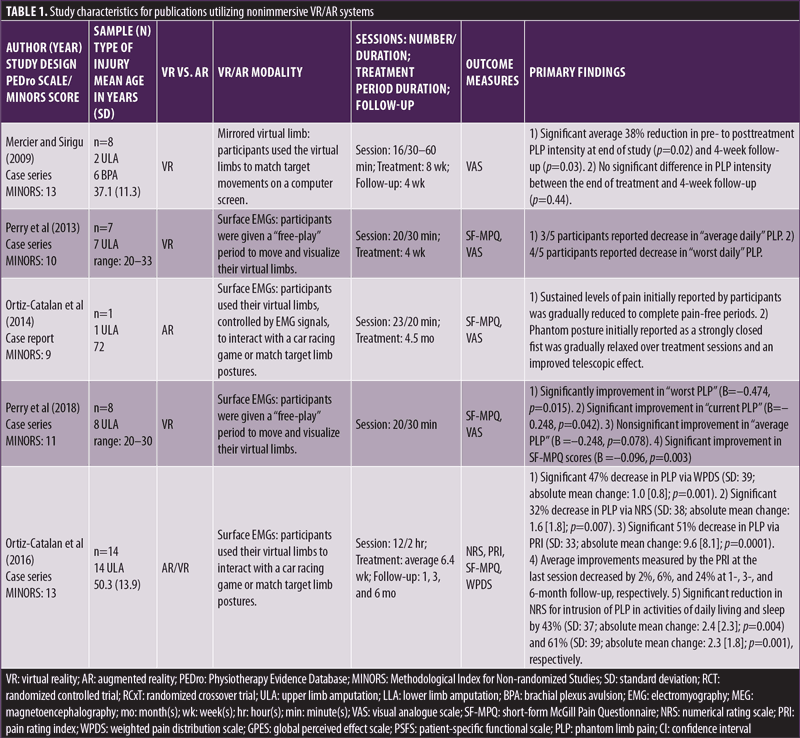
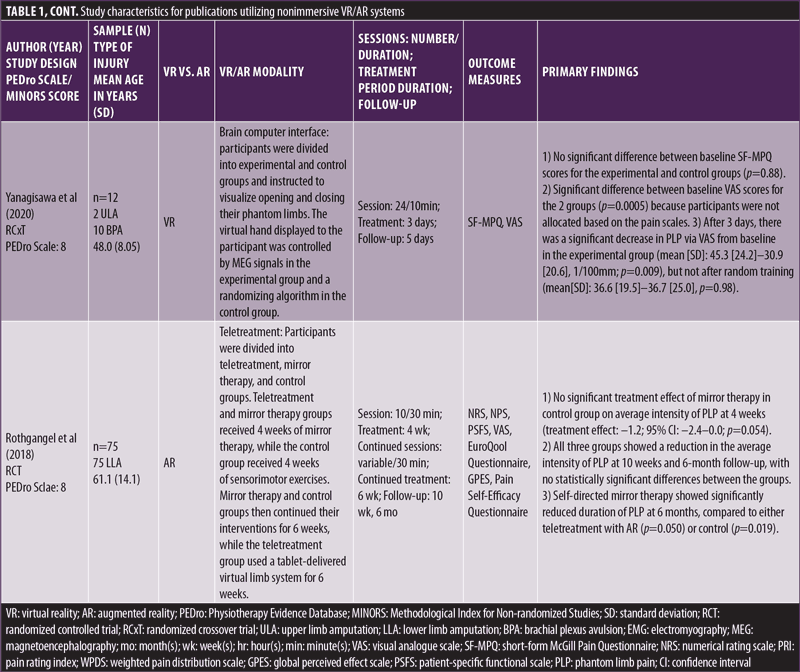
Mercier and Sirigu20 utilized a mirrored virtual limb system in which movements of the intact limb were recorded, reflected, and displayed to the participant as a virtual representation of the affected limb. Perry et al21,22 and Ortiz-Catalan et al23,24 utilized a system of surface electromyographies (EMGs) on the residual limb to capture neuroelectrical signals and interpret them to control a virtual limb. The RCxT by Yanagisawa et al19 created a unique VR system consisting of a brain-computer interface (BCI) capturing magnetoenceophalography (MEG) signals directly from the sensorimotor cortex. As participants were instructed to think of opening and closing their affected hand, the BCI would interpret MEG signals to control the opening or closing of a virtual hand.19 The RCT by Rothgangel et al18 divided participants into teletreatment, MT, and control groups. The teletreatment and MT groups received four weeks of traditional MTs, while the control group received four weeks of sensorimotor exercises. After the four-week period, participants in the teletreatment group underwent six weeks of tablet-based therapies, including an AR system which superimposed a virtual limb on top of the participants residual limb. The MT group and control group continued six weeks of traditional, self-delivered MT and sensorimotor exercises, respectively.18
All the nonimmersive studies reported improvements in PLP intensity, regardless of AR/VR implementation (Table 1). Specifically, studies by Yanagisawa et al,19 Mercier and Sirigu,20 Perry et al,22 and Ortiz-Catalan et al24 reported significant reductions in PLP intensity after the conclusion of the intervention period.While the teletreatment, MT, and control groups in the RCT by Rothgangel et al18 each reported decreased PLP intensity, there were no significant differences between the three groups. Follow up analysis performed by Rothgangel et al,18 Yanagisawa et al,19 Mercier and Sirigu,20 and Ortiz-Catalan et al24 found no significant changes in PLP intensity at the conclusion of the intervention period.
Immersive studies. Thirteen of the 20 included studies met the criteria for an immersive VR or AR intervention. Three were comparative case series,34–36 nine were noncomparative case series,12,25–32 and one was a case report,33 with average MINORS scores of 17.3, 10.6, and 8.0, respectively. Various VR implementations were used, including creating the virtual limb from a mirror image of the intact limb,25–29 physically controlling the virtual limb with the residual limb via external fixations,12,30 controlling the virtual limb via electromagnetic sensors,31–33 and VR systems that implemented tactile sensations.34–36 General characteristics of the nonimmersive studies were extracted and synthesized in Table 2.
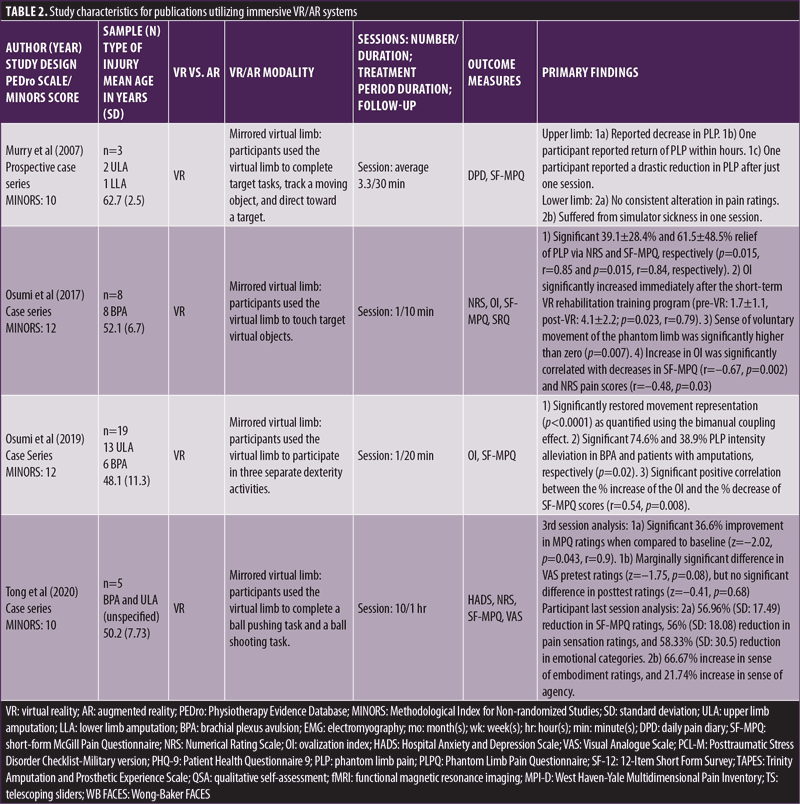
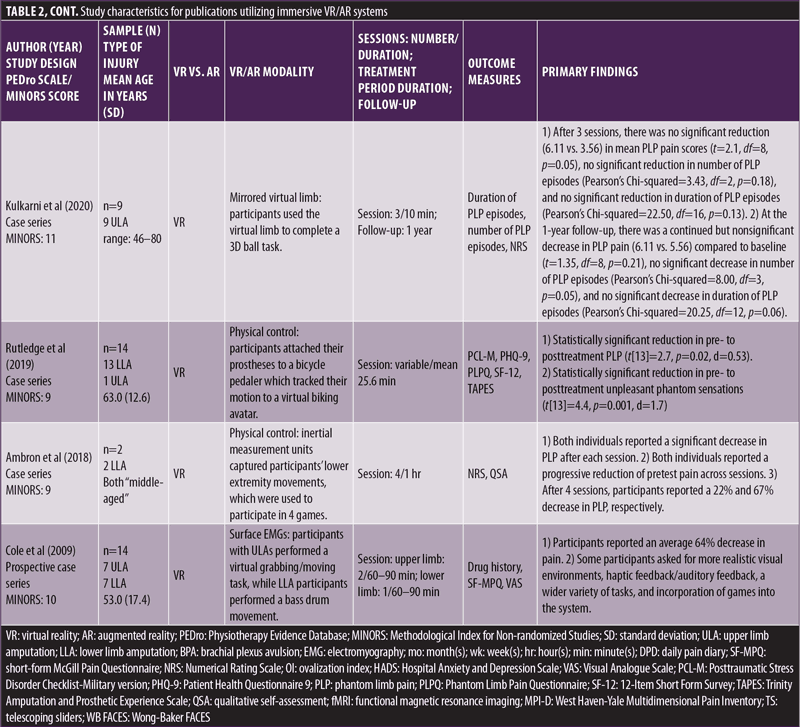
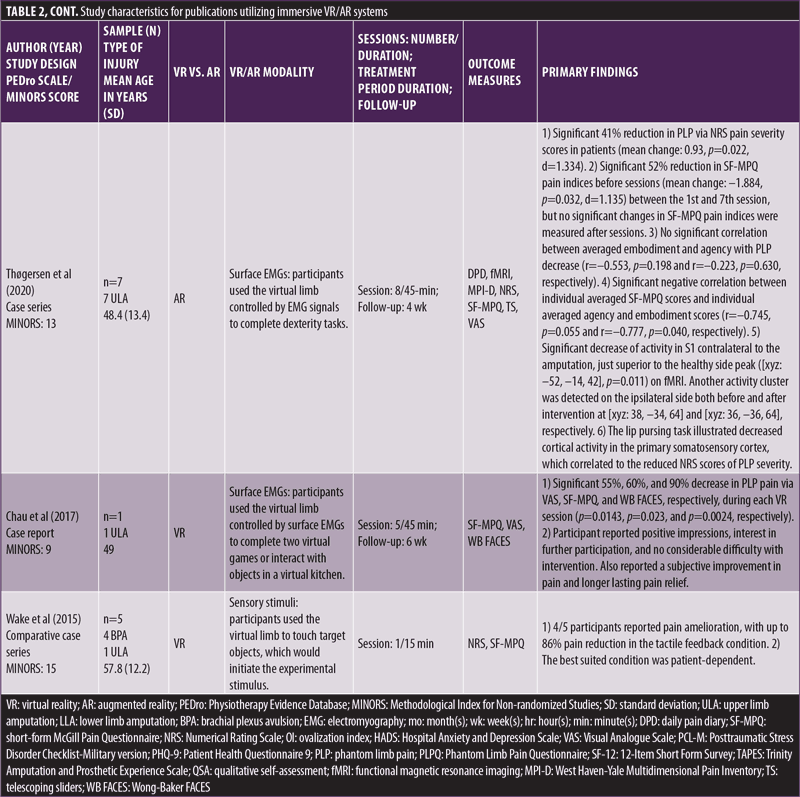
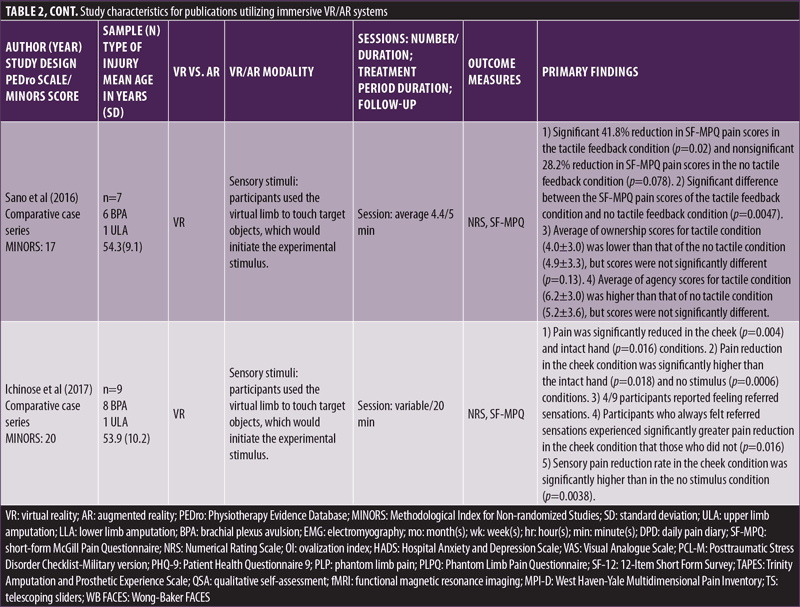
Studies by Murry et al,25 Osumi et al,26,27 Tong et al,28 and Kulkarni et al29 utilized a mirrored virtual limb system to create their virtual environments in which participants used their virtual limbs to interact and complete tasks specific to each study. Rutledge et al12 and Ambron et al30 relied on direct physical control by the amputated limb to control a virtual avatar. Rutledge et al specifically utilized a pedaling machine with motion sensors that interpreted and displayed participants riding a virtual bike.12 Ambron et al captured lower extremity movements with a series of inertial measurement units (IMUs) attached to participants during various scenarios.30 Studies by Cole et al,31 Thøgersen et al,32 and Chau et al33 relied on electromagnetic sensors attached to the residual limb to provide signals to control a virtual limb. Additionally, Thøgersen et al also attempted to measure the extent of cortical reorganization of lip representation into limb cortices by performing baseline and postintervention functional magnetic resonance imaging (fMRI) while participants performed a lip pursing task.32 Wake et al,34 Sano et al,35 and Ichinose et al36 utilized a unique VR system that took advantage of the phenomenon of illusory perceptions in PLP reported by Ramachandran et al.37,38 The latter documented the finding that when individuals with PLP were touched on their ipsilateral cheek, shoulder, or limb, they felt as if their phantom limb had been touched. Following up on this phenomenon, these studies provided various combinations of tactile stimuli to these referred locations to enhance the VR environment with tactile sensations, compared to a VR environment without tactile sensations.
All immersive studies reported a decrease in PLP by participants in the experimental groups (Table 2). Specifically, studies by Rutledge et al,12 Osumi et al,26,27 Tong et al,28 Thøgersen et al,32 Chau et al,33 Sano et al,35 and Ichinose et al36 reported significant decreases in PLP by participants in the experimental groups. Follow-up analysis done by Kulkarni et al29 at one year found no significant changes in PLP from baseline. Follow-up by Thøgersen et al32 and Chau et al at four and six weeks, respectively, found no significant changes in PLP from the conclusion of the interventional period, but participants in Chau et al33 reported decreased and more tolerable PLP from baseline.
In addition to measurements of PLP, Osumi et al26,27 utilized a bimanual circle test (BCT) to determine an ovalization index (OI) as an indicator for voluntary control of the phantom limb. In the 201726 and 201927 studies, Osumi et al found a significant increase in OI scores after intervention (p=0.023 and p<0.0001, respectively) and a significant correlation between the decrease in SF-MPQ pain intensity and increase in OI (r=–0.65, p=0.03 and r=0.54, p=0.008, respectively). Additionally, the 2019 study found a novel, significantly greater alleviation of PLP in participants with PLP secondary to BPA when compared to participants with PLP secondary to amputation (p=0.02).27
Under the PLP theory of cortical reorganization, the attempt by Thøgersen et al to measure the extent of cortical reorganization with fMRI found significant activations in the contralateral S1 Brodmann area 3b in both pre- and postintervention. However, when comparing the pre- and postintervention fMRI results, there was a significant reduction in activity in the contralateral S1 region (p=0.011). Plots comparing improvement of PLP severity to fMRI signals indicated a possible association of PLP severity and cortical reorganization. However, due to the small sample size involved in this study, no statistical correlation was performed.32
In the comparisons between tactile versus no tactile feedback to VR interventions, Sano et al35 found a significant decrease in the tactile feedback condition (p=0.02), with a significant difference from the no tactile feedback condition (p=0.0047). Ichinose et al36 reported a similar finding in which tactile stimulus to the ipsilateral cheek resulted in significantly reduced PLP, compared to the control hand condition (p=0.018) and control no stimuli condition (p=0.0006).
Discussion
The aim of this review was to perform an up-to-date systematic review of the growing number of studies researching AR/VR therapies for the treatment of PLP. These findings were used to create a succinct table of each study’s methodology, results, and summarized findings. In both nonimmersive and immersive AR/VR modalities, the majority of studies revealed a significant reduction in PLP, demonstrating that VR and AR therapies continue to be a developing and potentially promising option for the treatment of PLP. In 2017, when Dunn et al13 performed a literature review on the same topic, only eight studies that showed evidence of improvements in PLP were available, but they had limited statistical analysis or power. However, only four years later, 12 more studies have been performed, bringing the total to 20 studies. Out of the 20 studies, 18 were case series or case reports, typically with small sample sizes and lack of a control condition. However, while the eight studies before 2017 often utilized qualitative data without data analysis, the 12 more recent studies used increasing amounts of quantitative data with more robust investigations. Additionally, these more recent studies have expanded upon the immersion, customization, outcome measurement, and statistical analysis of VR and AR therapies for PLP. The greater number of immersive studies versus nonimmersive studies also alludes to the increase in availability and decrease in cost of the immersive VR systems utilized, mitigating a potential barrier to the clinical use of VR therapeutics.
Calabrò et al39 evaluated motor patterns using electroencephalogram (EEG) with robotic nonimmersive VR therapies. The findings of that research suggested that increased immersion of realistic tasks in VR provided increased frontoparietooccipital and mirror neuron system (MNS) activation.39 While increased participant interest and engagement in VR therapies play a role in the findings of the included studies, we also hypothesize that the increased frontoparietoccipital and MNS activation from the multisensory engagement of VR therapies might trigger a reduction in the pathologic cortical reorganization underlying PLP. However, while this systematic review indicates increasing evidence for improvements in PLP using AR/VR related therapies, there continues to be viable research avenues by which further exploration on benefits of AR/VR therapies for PLP may be undertaken.
Limitations. This review was limited by several factors related to the included studies’ methodologies and outcome measures. While many of the included studies had overlaps in methodology and primary outcome measures, there was a large range of lengths of treatments, number of treatments, and specific PLP scales used in the studies. This heterogeneity in methodologies limits the ability to draw specific conclusions about treatment benefits across the studies. Additionally, while there has been an increase in statistical measurements in the studies, many studies continue to have small sample sizes, and results should be interpreted with caution. Although these reasons limit conclusions drawn from these studies, we saw an improvement in addressing these concerns with more recent studies. These limitations continue to be areas requiring further research into treatment implications. Future studies could also benefit from further comparisons to other therapies used to treat PLP, including the optimal length and number of AR/VR treatments, and larger sample sizes with randomization in treatment assignments.
Conclusion
AR/VR therapies continue to be a developing field for the treatment of PLP. In recent years, there has been an increase in investigations studying its effectiveness, as well as more robust data analysis. This increase has paralleled the development of VR systems marketed for entertainment to the consumer audience.40 While AR/VR systems and methodology vary widely in the available literature, there continues to be evidence that AR/VR therapies are a promising and relatively safe treatment to reduce PLP. Future investigations could benefit from an increased focus on comparisons of AR/VR-based therapies against other available PLP treatments by optimizing number and length of sessions, as well as increasing sample sizes and randomization.
References
- Barbin J, Seetha V, Casillas JM, et al. The effects of mirror therapy on pain and motor control of phantom limb in amputees: a systematic review. Ann Phys Rehabil Med. 2016;59(4):270–275.
- Aternali A, Katz J. Recent advances in understanding and managing phantom limb pain. F1000Res. 2019;8: F1000 Faculty Rev-1167.
- McCormick Z, Chang-Chien G, Marshall B, et al. Phantom limb pain: a systematic neuroanatomical-based review of pharmacologic treatment. Pain Med. 2014;15(2):292–305.
- Kuffler DP. Coping with phantom limb pain. Mol Neurobiol. 2018;55(1):70–84.
- Collins KL, Russell HG, Schumacher PJ, et al. A review of current theories and treatments for phantom limb pain. J Clin Invest. 2018;128(6):2168–2176.
- Flor H, Elbert T, Knecht S, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375(6531):482–484.
- Urits I, Seifert D, Seats A, et al. Treatment strategies and effective management of phantom limb-associated pain. Curr Pain Headache Rep. 2019;23(9):64.
- Alviar MJM, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016;10:CD006380.
- McCabe C. Mirror visual feedback therapy. A practical approach. J Hand Ther. 2011;24(2):170–178; quiz 179.
- Rothgangel A, Bekrater-Bodmann R. Mirror therapy versus augmented/virtual reality applications: towards a tailored mechanism-based treatment for phantom limb pain. Pain Manag. 2019;9(2):151–159.
- Bernardo A. Virtual reality and simulation in neurosurgical training. World Neurosurg. 2017;106:1015–1029.
- Rutledge T, Velez D, Depp C, et al. A virtual reality intervention for the treatment of phantom limb pain: development and feasibility results. Pain Med. 2019;20(10):2051–2059.
- Dunn J, Yeo E, Moghaddampour P, et al. Virtual and augmented reality in the treatment of phantom limb pain: a literature review. NeuroRehabilitation. 2017;40(4):595–601.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–133.
- Cashin AG, McAuley JH. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J Physiother. 2020;66(1):59.
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716.
- Rothgangel A, Braun S, Winkens B, et al. Traditional and augmented reality mirror therapy for patients with chronic phantom limb pain (PACT study): results of a three-group, multicentre single-blind randomized controlled trial. Clin Rehabil. 2018;32(12):1591–1608.
- Yanagisawa T, Fukuma R, Seymour B, et al. BCI training to move a virtual hand reduces phantom limb pain: a randomized crossover trial. Neurology. 2020;95(4):e417–e426.
- Mercier C, Sirigu A. Training with virtual visual feedback to alleviate phantom limb pain. Neurorehabil Neural Repair. 2009;23(6):587–594.
- Perry BN, Mercier C, Pettifer SR, et al. Virtual reality therapies for phantom limb pain. Eur J Pain. 2014;18(7):897–899.
- Perry BN, Armiger RS, Wolde M, et al. Clinical trial of the virtual integration environment to treat phantom limb pain with upper extremity amputation. Front Neurol. 2018;9770.
- Ortiz-Catalan M, Sander N, Kristoffersen MB et al. Treatment of phantom limb pain (PLP) based on augmented reality and gaming controlled by myoelectric pattern recognition: a case study of a chronic PLP patient. Front Neurosci. 2014;8:24.
- Ortiz-Catalan M, Guðmundsdóttir RA, Kristoffersen MB, et al. Phantom motor execution facilitated by machine learning and augmented reality as treatment for phantom limb pain: a single group, clinical trial in patients with chronic intractable phantom limb pain. Lancet. 2016;388(10062)2885–2894.
- Murray CD, Pettifer S, Howard T, et al. The treatment of phantom limb pain using immersive virtual reality: three case studies. Disabil Rehabil. 2007;29(18):1465–1469.
- Osumi M, Ichinose A, Sumitani M, et al. Restoring movement representation and alleviating phantom limb pain through short-term neurorehabilitation with a virtual reality system. Eur J Pain. 2017;21(1):140–147.
- Osumi M, Inomata K, Inoue Y, et al. Characteristics of phantom limb pain alleviated with virtual reality rehabilitation. Pain Med. 2019;20(5):1038–1046.
- Tong X, Wang X, Cai Y, et al. “I dreamed of my hands and arms moving again”: a case series investigating the effect of immersive virtual reality on phantom limb pain alleviation. Front Neurol. 2020;11:876.
- Kulkarni J, Pettifer S, Turner S, Richardson C. An investigation into the effects of a virtual reality system on phantom limb pain: a pilot study. Br J Pain. 2020;14(2):92–97.
- Ambron E, Miller A, Kuchenbecker KJ, et al. Immersive low-cost virtual reality treatment for phantom limb pain: evidence from two cases. Front Neurol. 20189:67.
- Cole J, Crowle S, Austwick G, Slater DH. Exploratory findings with virtual reality for phantom limb pain; from stump motion to agency and analgesia. Disabil Rehabil. 2009;31(10):846–854.
- Thøgersen M, Andoh J, Milde C, et al. Individualized augmented reality training reduces phantom pain and cortical reorganization in amputees: a proof of concept study. J Pain. 2020;21(11–12):1257–1269.
- Chau B, Phelan I, Ta P, et al. Immersive virtual reality therapy with myoelectric control for treatment-resistant phantom limb pain: case report. Innov Clin Neurosci. 2017;14(7–8):3–7.
- Wake N, Sano Y, Oya R, et al. Multimodal virtual reality platform for the rehabilitation of phantom limb pain. 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER). 2015:787–790.
- Sano Y, Wake N, Ichinose A, et al. Tactile feedback for relief of deafferentation pain using virtual reality system: a pilot study. J Neuroeng Rehabil. 2016;13(1):61.
- Ichinose A, Sano Y, Osumi M, et al. Somatosensory feedback to the cheek during virtual visual feedback therapy enhances pain alleviation for phantom arms. Neurorehabil Neural Repair. 2017;31(8):717–725.
- Ramachandran VS, Rogers-Ramachandran D. Phantom limbs and neural plasticity. Arch Neurol. 2000;57(3):317–320.
- Ramachandran VS, Hirstein W. The perception of phantom limbs. The D. O. Hebb lecture. Brain. 1998;121(Pt 9):1603–1630.
- Calabrò RS, Naro A, Russo M, et al. The role of virtual reality in improving motor performance as revealed by EEG: a randomized clinical trial. J Neuroeng Rehabil. 2017;14(1):53.
- The Economist. Headset technology is cheaper and better than ever. 1 Oct 2020. https://www.economist.com/technology-quarterly/2020/10/01/headset-technology-is-cheaper-and-better-than-ever. Accessed 20 Sep 2022.


