 by Masaru Nakamura, MD, PhD, and Takahiko Nagamine, MD, PhD
by Masaru Nakamura, MD, PhD, and Takahiko Nagamine, MD, PhD
Dr. Nakamura is with the Department of Psychiatric Internal Medicine, Kosekai-Kusatsu Hospital, Hiroshima, Japan; and Dr. Nagamine is with the Department of Psychiatric Internal Medicine, Shinseikai-Ishii Memorial Hospital, Iwakuni, Japan
Innov Clin Neurosci. 2015;12(9–10):18–24.
Funding: No funding was received for the preparation of this manuscript.
Financial Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Key words: Levocarnitine, valproic acid, hyperammonemia, carnitine deficiency, psychiatric setting
Abstract: Objective: The aim of this study was to investigate the effect of levocarnitine (active isoform of carnitine, L-Carnitine) supplementation on serum ammonia and carnitine levels simultaneously, and their clinical outcomes in valproic acid-treated psychiatric subjects. Design: This was a propsective study of 22 psychiatric patients. Method: A fixed dose of levocarnitine was coadministrated over a period of three months in subjects with valproic acid-induced hyperammonemia. Serum ammonia, valproic acid, and carnitine concentrations were measured, and psychiatric symptoms were recorded at baseline and one, two, and three months. Sequential change of the levels of serum ammonia, valproic acid, free carnitine, acylcarnitine, total carnitine, ratios of acylcarnitine/free carnitine, and the scores of Brief Psychotic Rating Scale, Clinical Global Impression-Severity scale, and the Global Assessment of Functioning scale were compared within total subjects and two groups divided by the effectiveness of levocarnitine supplementation against baseline hyperammonemia. Results: Within total subjects, free carnitine, acylcarnitine, and total carnitine levels were significantly increased without statistical change in serum ammonia, valproic acid levels, and acylcarnitine/free carnitine ratio, while a part of Brief Psychotic Rating Scale scores was decreased. Acylcarnitine levels were significantly increased at all points in the levocarnitine effective group and not in the noneffective group. Acylcarnitine/free carnitine ratio increased in the levocarnitine effective group and decreased in the noneffective group, which was confirmed by negative correlation between the ratio of serum ammonia levels at three months to serum ammonia levels at baseline and the ratio of acylcarnitine/free carnitine levels at three months to acylcarnitine/free carnitine levels at baseline. Conclusion: In valproic acid-treated psychiatric patients, carnitine supplementation resulted in overall improvement in mental status. Improvement of hyperammonemia and carnitine deficiency in this group may be related to mitochondrial function.
Introduction
Valproic acid (valproate, VPA) is a branched, medium-chain fatty acid agent having a broad-spectrum antiepileptic action. In the psychiatry clinical setting, VPA is frequently used for several psychiatric disorders as a mood stabilizer and combined with various types of antipsychotics.[1] Adverse reactions to VPA involve neurological, hematopoietic, hepatic, and digestive systems.[2] Among the metabolic disorders, VPA-induced hyperammonemia,[3] which is characterized by lethargy, vomiting, cognitive slowing, focal neurological deficits, and decreasing consciousness ranging from drowsiness to coma, is an important clinical consideration.3 In psychiatric patients, VHE may be difficult to differentiate from the exacerbation of psychiatric symptoms. The exact mechanism of VHE remains unclear but is believed to be related to the accumulation of toxic VPA metabolites and elevated ammonia (NH3) levels.[4]
Carnitine is an essential cofactor in the proper metabolism of VPA and NH3 elimination. A severe carnitine deficiency is thought to contribute to VHE.[5] Although the precise mechanism leading to hypocarnitinemia in patients treated with valproate is not known, a previous clinical study showed a significant decrease in carnitine concentration and changes in the ratio of acylcarnitine to free carnitine for both neurological and psychiatric indications.[6] Recently, we reported that VPA treatment induces hyperammonemia and causes concentration-dependent carnitine deficiency regardless of the use of additional mood stabilizers, while serum levels of NH3 do not correlate with VPA concentrations in psychiatric patients.[7]
In the field of pediatric neurology, reports suggests carnitine supplementation tends to normalize elevated NH3 concentration by binding to VPA and relieving the inhibition of urea synthesis with favorable clinical response.[8,9] In contrast, despite the widespread use of VPA in psychiatry, carnitine supplementation for the treatment of hyperammonemia and hypocarnitinemia has not been prospectively studied in a psychiatric setting. The aim of our present study was to investigate the effect of levocarnitine (active isoform of carnitine, L-Carnitine) supplementation on serum NH3 and carnitine levels and how its use may impact clinical outcomes in VPA-treated psychiatric subjects.
Methods
Subjects. Psychiatric patients treated with VPA continuously for three months or more with high plasma NH3 concentration (>86?g/dL) were selected. Use of antipsychotic medication and/or concomitant use of mood stabilizers was permitted. Diagnoses of mental and behavioral disorders were made according to the International Statistical Classification of Disease and Related Health Problems, 10th Revision (ICD-10). The exclusion criteria included undergoing treatment of hyperammonemia, receiving supplemental carnitine, and presence of medical conditions that directly affect serum NH3 or carnitine levels (e.g., hepatic or renal failure). Under these criteria, subjects who agreed to levocarnitine supplementation with VPA treatment were enrolled consecutively and studied prospectively from September 2013 to June 2014 at Kusatsu Hospital in Hiroshima, Japan. Study subjects consisted of 15 men (12 with schizophrenia/schizoaffective disorder and 3 with bipolar disorder) with a mean age of 51 years and seven women (4 with schizophrenia/schizoaffective disorder, one with bipolar disorder, and two with epilepsy/posttraumatic head injury) with a mean age of 48 years.
The ethics committee of Kusatsu Hospital approved this study, and written informed consent was obtained from all participants.
Procedures. All subjects were administrated fixed doses of levocarnitine (30mg/kg body weight) in addition to their fixed doses of VPA throughout the three-month observation period. As we have previously described,[7] the following samples and psychiatric symptoms were collected/assessed and measured at baseline, and one, two, and three months:
Fasting plasma samples for analysis of NH3 and carnitine levels were drawn at the same time as samples for VPA concentrations (?g/mL) and immediately frozen for subsequent measurement in all subjects. Serum NH3 concentrations were measured by the colorimetric method (FUJI Dry-Chem 5500).[10] Serum carnitine was evaluated separately as free carnitine (FC) and acylcarnitine (AC) by the chromatography-tandem mass spectrometry (API 3000; Applied Biosystems, Foster City, California, USA).[11] Total carnitine (TC) was calculated by adding FC and AC, and the ratio of AC to FC (AC/FC) was assessed.
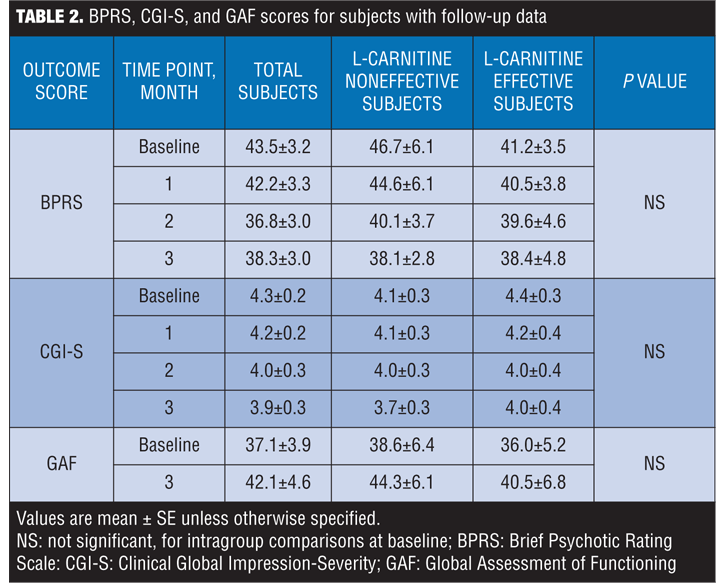
Psychiatric symptoms were evaluated using the 18-item Brief Psychotic Rating Scale (BPRS)[12] and the Clinical Global Impression-Severity (CGI-S)[13] scale at baseline, and one, two, and three months, and the Global Assessment of Functioning (GAF) scale at baseline and three months.
Outcome measures and statistical methods. In all subjects, multiple comparisons of the measured values at baseline and one, two, and three months were performed with repeated-measured ANOVA, followed by post-hoc analysis using Boneferroni adjustment, and considered statistically significant at P<0.05.
After the values of NH3 were obtained, we retrospectively divided subjects into two groups according to their variation; NH3-reduced samples without an increase from baseline throughout three months were classified as the levocarnitine effective group (effective group; 9 men and 3 women), and other samples such as NH3-unchanged or increased samples were the levocarnitine noneffective group (noneffective group; 6 men and 4 women). Baseline differences of the levels of NH3, VPA, FC, AC, TC, and AC/FC ratios between the levocarnitine effective and noneffective groups were evaluated by unpaired, Mann-Whitney U test, and considered statistically significant at p<0.05. Within each subgroup, multiple comparisons of the measured values at baseline and one, two, and three months were also performed.
Among all subjects, the data were analyzed to see whether there is any significant correlation between the ratio of three-momths NH3 to baseline NH3 level and the ratio of the three-months AC/FC to baseline AC/FC, by the Spearman’s correlation coefficient method and considered statistically significant at p<0.05.
Results
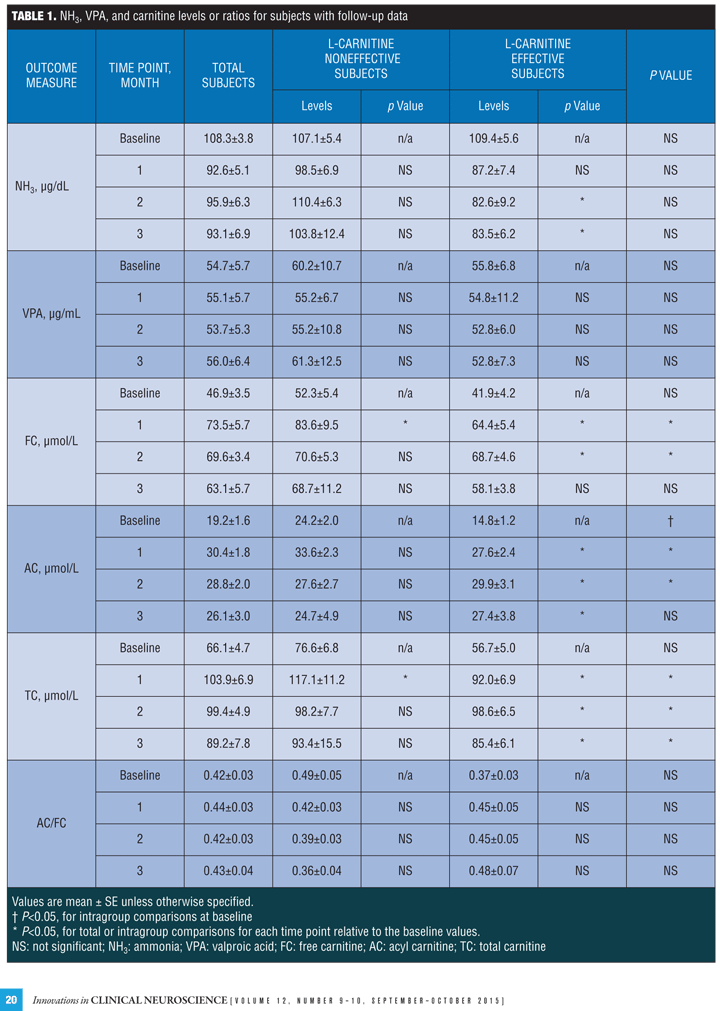
NH3, VPA and carnitine levels or ratios in total subjects. The levels of NH3 were decreased but not significantly and VPA were almost not changed from the baseline. The levels of FC and AC were significantly increased at one and two month and the levels of TC were significantly increased at all observed points from baseline, while the AC/FC ratios remained unchanged.
NH3, VPA, and carnitine levels or ratios in effective and noneffective subjects. At baseline, AC levels in the effective group were significantly higher than those in the noneffective group, while no differences were found in the levels of NH3, VPA, FC, TC, and AC/FC ratios.
NH3 levels were significantly decreased at two and three month from baseline in the effective group but not in the noneffective group at any point, and VPA levels were stable in both groups.
FC levels were significantly increased at one and two months from baseline in the effective group and at one month in the noneffective group. AC levels were significantly increased at all points from baseline in the effective group and not statistically increased at any point in the noneffective group. TC levels were significantly increased at one, two and three months from baseline in the effective group and at one month in the noneffective group. The AC/FC ratios increased in the effective group and decreased in the noneffective group at any point after baseline, although not significantly.
The changes of the values over time from baseline to one, two, and three months are presented in Table 1, and those in the effective and noneffective groups are separately extracted from the database as
Figure 1.
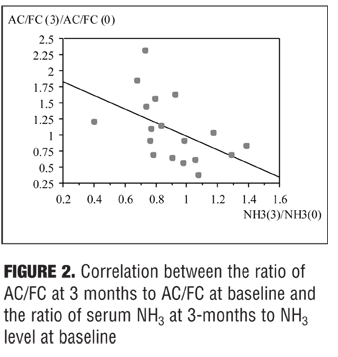
The ratio of AC/FC levels at three months to AC/FC levels at baseline was significantly negatively correlated with the ratio of NH3 levels at three months to NH3 levels at baseline
(r= -0.497, p=0.0359) as shown in Figure 2.
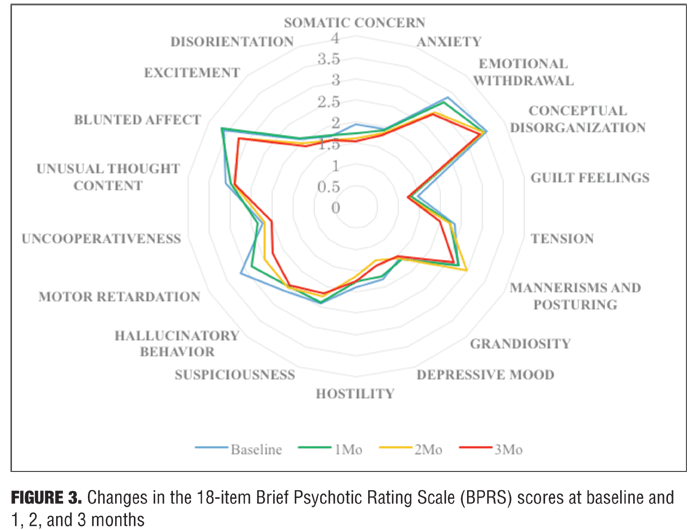
BPRS, CGI-S, and GAF scores in subjects. The total scores on the BPRS and the CGI-S scores decreased, not significantly, at one, two, and three months from baseline. There was no significant change in the GAF scores. Statistically significant improvement was observed from baseline to three months for one of the 18 BPRS items—motor retardation. These psychiatric-related signs are presented in the form of Table 2 and Figure 3.
Discussion
The results of the present study could be summarized as follows: 1) levocarnitine supplementation led to an increase in FC, AC and TC levels, irrespective of an improvement of hyperammonemia, 2) levocarnitine add-on therapy was effective in reducing high plasma NH3 concentrations in about half of the subjects with VPA-induced hyperammonemia, 3) baseline levels of AC and longitudinal changes of AC/FC ratios were correlated with fluctuation of NH3 levels from baseline, 4) levocarnitine treatment did not generally exacerbated mental status, but ameliorated a part of psychiatric symptoms.
The two main metabolic functions of carnitine are to facilitate fatty acyl group transport into mitochondria and to maintain the ratio of acyl-CoA to free CoA in the mitochondria:[14] 1) Carnitine is an essential amino acid necessary for the beta-oxidation of fatty acids and energy production in mitochondria. Under normal metabolic conditions, acyl-CoA is transported into mitochondria via a carnitine transport system. It is then converted to acetyl-CoA via beta-oxidation and eventually to N-acetyl glutamate; and 2) Carnitine facilitates prevention of intramitochondrial accumulation of acyl-CoA by transforming acyl-CoA into acylcarnitine. In this way, carnitine protects the cell from the membrane-destabilizing effects of toxic acyl groups. Carnitine thus plays a central role in the metabolism of fatty acids and energy by regulating the mitochondrial ratio of free CoA to acyl-CoA. This pathway can be interrupted by the introduction of VPA, which leads to a reduction of free coenzyme A, acetyl-CoA, and carnitine, and results in the decreased availability of cofactors necessary for the function of urea cycle and an increase in NH3 production.[15,16]
Because VPA-induced metabolic disorders including hyperammonemia could be mediated at least in part by carnitine deficiency, it has been hypothesized that levocarnitine supplementation may prevent, correct, or attenuate these adverse effects[15,17] Several studies or isolated clinical cases have suggested the potential value of oral levocarnitine in reversing carnitine deficiency or preventing adverse effects due to VPA-induced dysfunction of beta-oxidation.
Böhles et al8 investigated the effects of carnitine supplementation in 15 (14 hyperammonemic) children with epilepsy treated with VPA monotherapy, and found that the prolonged L-carnitine supplementation was associated with normalization in plasma NH3 concentrations and marked increase in carnitine concentration in all patients with the significant correlations between the plasma NH3 concentrations and the AC/FC ratio. Raskind et al[18] extensively reviewed the pathophysiology and significance of VPA-induced carnitine deficiency and evaluated the literature pertaining to carnitine supplementation during VPA therapy in children, and concluded that despite the lack of prospective, randomized clinical trials, a few studies have shown carnitine supplementation in patients receiving VPA result in subjective and objective improvements along with increases in carnitine serum levels. A few sporadic observations also suggest that L-carnitine may be useful in patients with coma or in preventing hepatic dysfunction after acute VPA overdose.[19,20]
Thus carnitine supplementation in high-risk patients, especially in pediatrics or in acute overdose phase during VPA therapy are now recommended by some scientific committees and textbooks;[21] however, few articles have been published on the efficacy of levocarnitine supplementation in chronic VPA-treated psychiatric patients.
Cutiric et al[22] surveyed clinical outcomes in psychiatric patients with documented hypocarnitinemia who were receiving oral levocarnitine supplementation by a retrospective chart view method, and found that, initially, some degree of cognitive impairment improved in most of the patients after correction of carnitine serum levels. They also compared the selected parameters at initial presentation and at end of follow-up period and found that initial low carnitine (FC, AC, and TC) levels were statistically significantly higher at the end of the follow-up, while there were no differences in AC/FC ratio and venous blood NH3 levels.
In our prospectively designed study investigating the subjects as a whole, the levels of carnitine were significantly increased at any observed point from baseline, but no statistical changes were observed in NH3 and VPA levels and in AC/FC ratio. In addition, we retrospectively divided the obtained outcomes into two groups according to the longitudinal change pattern of NH3 levels and investigated them within each group. The most notable finding was the contrary trends in respect of AC/FC ratio between the levocarnitine effective and noneffective groups, which was confirmed by significant correlation between the ratios of end point AC/FC levels to baseline AC/FC levels and the ratio of end point NH3 levels to baseline NH3 levels. Administration of exogenous carnitine as an adjunct to VPA therapy is thought to restore beta-oxidation to mitochondria cells of the liver, accept toxic acyl moieties from CoA, and relieve the inhibition of urea synthesis.[3] However, our findings suggested that the effective utilization of levocarnitine transport into mitochondria, which may be individually different, might play an important role to lead these favorable processes.
Mitochondrial dysfunction results from alterations in biochemical cascade, and the damage to the mitochondrial electron transport chain has been suggested to be an important factor in the pathogenesis of a range of neuropsychiatric disorders, such as bipolar disorder, depression, and schizophrenia.[23–25] The etiology and pathophysiology of schizophrenia remain unknown, but alterations of mitochondrial oxidative phosphorylation in schizophrenia have been reported in several brain regions and also in platelets, and abnormal mitochondrial morphology, size, and density have all been reported in the brains of individuals with schizophrenia.[26,27] These findings may support our hypothesis that individual variation of mitochondrial function is associated with the effectiveness of carnitine supplementation on hyperammonemia and carnitine metabolism treated with VPA in psychiatric patients.
Limitations. There are several limitations to the present study. First, the sample size was small and resulted in low statistical power. Second, the observed duration of carnitine supplementation was limited to three months. Third, we did not take into account the concentration of VPA in each subject when we determined the administrated dose of carnitine.
Conclusion
Despite the limitations, our findings suggest that carnitine supplementation results in overall improvement of mental status, and that improvement of hyperammonemia and hypocarnitinemia are related to mitochondrial function in VPA-treated psychiatric patients.
Optimal dosage and length of treatment with carnitine supplementation in VPA-induced hyperammonemia and hypocarnitinemia, as well as predictability of carnitine deficiency-related symptoms, need to be clarified in future research to confirm our observation.
Acknowledgment
We would like to thank our colleagues at Kusatsu Hospital for their assistance with this report.
References
1. Stahl SM, Grady MM. A critical review of atypical antipsychotic utilization: comparing monotherapy with polypharmacy and augmentation. Curr Med Chem. 2004;11:313–327.
2. Gomceli YB, Kutlu G, Cavdar L, et al. Different clinical manifestations of hyperammonemic encephalopathy. Epilepsy Behav. 2007;10:583–587.
3. Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol. 2009;47:101–111.
4. Segura-Bruna N, Rodriguez-Campello A, Puente V, Roquer J. Valproate-induced hyperammonemic encephalopathy. Acta Neurol Scand. 2006;114:1–7.
5. Verrotti A, Trotta D, Morgese G, et al. Valproate-induced hyperammonemic encephalopathy. Metab Brain Dis. 2002;17:367–373.
6. Moreno?FA,?Macey H, Schreiber B. Carnitine levels in valproic acid-treated psychiatric patients: a cross-sectional study. J Clin Psychiatry. 2005;66:555–558.
7. Nakamura M, Nagamine T. Hyperammonemia and carnitine deficiency treated with sodium valproate in psychiatric setting. Int Med J. 2015;22:132–135.
8. Böhles H, Sewell AC, Wenzel D. The effect of carnitine supplementation in valproate-induced hyperammonaemia. Acta Paediatr. 1996;85:446–449.
9. Mock CM, Schwetschenau KH. Levocarnitine for valproic-acid-induced hyperammonemic encephalopathy. Am J Health Syst Pharm. 2012;69:35–39.
10. Makiuchi H, Terashima K. Rapid test of blood NH3 using FUJI Dri-Chem 100N. Journal of the Japan Society for Clinical Laboratory Automation. 1993;18:204–208.
11. Yamada K, Mushimoto Y, Yamaguchi S. Storage temperature and pH affect acylcarnitine and free carnitine value analyzed by MS/MS. Journal of the Japanese Society for Mass-screening. 2012;22:29–34.
12. Overall JE, Gorham DR. The Brief Psychiatric Rating scale. Psychologic Rep. 1962;10:799–812.
13. Guy W (ed). ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Heath, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976.
14. Roe CRM, Kahler SG, Kodo N, et al. Carnitine homeostatis in the organic acidurias. In: Tanaka KC (ed). Fatty Acid Oxidation: Clinical, Biochemical, and Molecular Aspects. New York: Alan R Liss;1990:382–402.
15. Aires CC, van Cruchten A, Silva MF, et al. New insights on the mechanisms of valproate-induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol. 2011;55:426–434.
16. Nakajima Y, Ito T, Togari H, et al. Evaluation of valproate effects on acylcarnitine in epileptic children by LC-MS/MS. Brain Dev. 2011;33:816–823.
17. Lheureux PE, Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin Toxicol. 2009;47:101–111.
18. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34:630–638.
19. Ishikura H, Matsuo N, Tanaka T, et al. Valproic acid overdose and L-carnitine therapy. J Anal Toxicol. 1996;20:55–58.
20. Murakami K, Sugimoto T, Muro H, et al. Effect of L-carnitine supplementation on acute valproate intoxication. Epilepsia. 1996;37:687–689.
21. AACE Nutrition Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for the clinical use of dietary supplements and nutraceuticals. Endocr Pract.2003;9:417–470.
22. Cuturic M, Abramson RK, Moran RR, et al. Clinical outcomes and low-dose levocarnitine supplementation in psychiatric inpatients with documented hypocarnitinemia: a retrospective chart review. J Psychiatr Pract. 2010;16:5–14.
23. Iwamoto K, Bundo M, Kato T. Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum Mol Genet. 2005;14:261–253.
24. Fattal O, Budur K, Vaughan AJ, et al. Review of the literature on major mental disorders in adult patients with mitochondrial diseases. Psychosomatics. 2006;47:1–7.
25. Rezin GT, Amboni G, Zugno AI, et al. Mitochondrial dysfunction and psychiatric disorders. Neurochem Res. 2009;34:1021–1029.
26. Dror N, Klein E, Karry R, et al. State-dependent alterations in mitochondrial complex I activity in platelets: a potential peripheral marker for schizophrenia. Mol Psychiatry. 2002;7:995–1001.
27. Faizi M, Salimi A, Rasoulzadeh M, et al. Schizophrenia induces oxidative stress and cytochrome C release in isolated rat brain mitochondria: a possible pathway for induction of apoptosis and neurodegeneration. Iran J Pharm Res. 2014;13:93–100.





