
by Hoda A. Hussein, MD; Lamiaa I. Daker, MD; Nermeen A. Fouad, MD; Azza Elamir, MD; and Shereen R. Mohamed, MD
Dr. Hussein is with the Internal Medicine Department, Dr. Daker is with the Neurology Department, Dr. Fouad is with the Rheumatology Department, and Drs. Elamir and Mohamed are with the Medical Biochemistry Department—all with the Faculty of Medicine, Fayoum University, in Al Fayoum, Egypt.
Funding: No funding resources were declared for this research.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Innov Clin Neurosci. 2018;15(9–10):25–29
Abstract: Objective: Neurocognitive impairment is one of the most common systemic lupus erythematosus (SLE) manifestations. However, its pathophysiology is still poorly understood. Vitamin D deficiency is a possible risk factor for cognitive impairment. The aim of this study was to evaluate the relationship between 25-dihydroxy(OH) D3 levels and cognitive performance in patients with SLE. Methods: This was a cross-sectional, case-control study that included 30 Egyptian patients diagnosed with SLE and 20 age, sex, and educational level-matched controls. Study participants were subjected to a battery of neuropsychological evaluation using the California Verbal Learning Test (CVLT- II), Controlled Oral Word Association Test (COWAT), and Trail Making Test and evaluation of depression using Beck Depression Inventory (BDI). Serum levels of 25(OH) D3 were measured in the SLE group and control group. Results: The patients with SLE performed worse on total recall of verbal memory and executive function tests than the healthy controls. There was no significant difference between the patients and controls in Beck Depression Inventory (BDI). There was a significant negative correlation between vitamin D levels and executive function assessed by Trail Making Test (r=-0.399, p=0.03). Conclusion: Vitamin D deficiency could have a significant impact on cognitive performance in patients with SLE.
Keywords: Systemic lupus erythematosus, neuropsychiatric systemic lupus erythematosus, cognition, cognitive impairment, vitamin D
Systemic lupus erythematosus (SLE) is a chronic, systemic, relapsing-remitting autoimmune disease of unknown etiology with heterogeneous manifestations, including various neurologic and psychiatric syndromes. These neuropsychiatric syndromes typically fall under the term neuropsychiatric systemic lupus erythematosus (NPSLE).1 While neuropsychiatric manifestations associated with SLE are common, they are diverse in etiology and clinical presentation.
Cognitive impairment is prevalent among patients with SLE, but is heterogeneous in nature among this patient population.2 According to the American College of Rheumatology (ACR), cognitive impairment is present in 20 to 80 percent of patients with SLE.3,4 This wide variability is likely due to the demographic diversity of patients with SLE and the variety of psychometric tests used to measure cognitive dysfunction among these patients.2
Epidemiological studies have reported that vitamin D deficiency is a potential risk factor for cognitive impairment within the general population.11–13 Vitamin D is an essential steroid hormone that has a significant effect on mineral metabolism and skeletal health. More recently, vitamin D has been shown to have effects on cardiovascular and immune health.5 Researchers have also found an association between vitamin D levels and cognitive dysfunction in multiple disease states, such as Alzheimer’s disease, multiple sclerosis, and SLE.6–7,13
One group of researchers reported that vitamin D deficiency was associated with a substantially increased risk of dementia and Alzheimer’s disease among 1,658 elderly adults. 6 Another group of researchers observed a higher density of CA1 neurons in the middle regions of the hippocampus of aging rats that had been regularly administered calcitriol.8
In a cross-sectional analysis examining people with multiple sclerosis, investigators found a strong causal relationship between vitamin D levels and perceived cognitive impairment.7 It is thought that vitamin D enhances neuroprotection through its effects on proinflammatory cytokines, which contribute to cognitive decline and dementia.9
The sun is a common trigger for SLE symptoms but is also a primary source of vitamin D3; thus, patients with SLE are at a higher risk for vitamin D deficiency than the general population.10 Evidence indicates that vitamin D deficiency contributes to the disease activity and exacerbation of neuropsychiatric manifestations of SLE.14 The relationship between vitamin D and cognitive dysfunction in patients with SLE, however, has not been well studied. In the current study, we evaluated the relationship between 25(OH) D3 levels and cognitive performance in Egyptian patients with SLE.
Patients and Methods
Patients. This was a cross-sectional, case-control study that was approved by the ethics committee of the Faculty of Medicine at Fayoum University in Egypt. Written, informed consent was obtained from all participants. Study subjects were recruited during the period of April 2015 to May 2016 from Rheumatology Outpatient Clinic at Fayoum University Hospital. Thirty Egyptian patients diagnosed with SLE, according to the revised American College of Rheumatology (ACR) criteria for the classification of SLE (1997),15 were selected. Twenty age, sex, and education-level matched healthy volunteers with no history of neurologic or psychiatric conditions were selected as controls. The age of the participants ranged from 18 to 42 years. The mean duration of illness of the patients with SLE was 32.33± 29.61 months.
Exclusion criteria included the following:
- Presence of previous central nervous system (CNS) complications, such as lupus cerebritis, seizures, or focal neurological deficits
- Use of antipsychotic drugs or substances that might interfere with neuropsychological performance
- History of hypercalcemia, parathyroid dysfunction, treatment with diuretics, or renal impairment.
Methods. Subjects in both groups (SLE and control) underwent the following battery of neuropsychological testing, according to the Proposed American College of Rheumatology Neuropsychological Battery for Systemic Lupus Erythematosus (ACR-SLE), with 100-percent sensitivity and 94-percent specificity among non-NPSLE subjects.16
Assessment of verbal memory and learning ability was performed using the California Verbal Learning Test- 2nd edition (CVLT- II, Arabic edition).17 ,18 Assessment of verbal fluency was performed using the Controlled Oral Word Association Test (COWAT, Arabic edition).19 ,20 Executive functions assessment was performed using Trail Making Test (TMT-B, Arabic edition).21,22 Depression was evaluated using Beck Depression Inventory (BDI)23 (<10: normal; 11–16: mild depression).
Samples collection. Blood samples were obtained from patients with SLE and controls. A sample of their blood was collected into vacutainer ethylenediaminetetraacetic acid (EDTA) tubes to assess erythrocyte sedimentation rate (ESR) and complete blood count (CBC). Another sample was collected into a vacutainer tube without EDTA to assess liver enzymes (AST and ALT), serum creatinine, protein/creatinine ratio, presence of antinuclear antibodies (ANA), anti-double stranded deoxyribonucleic acid (anti-ds DNA) antibodies, and vitamin D. Urine samples were obtained from all participants to check for proteins, RBCs, and cellular casts for early diagnosis and follow-up of lupus nephritis.
Statistical analysis. Statistical Package for Social Science (SPSS) Version 16 was used for data management and analysis. The Mann-Whitney test was used for comparison of qualitative variables, while Spearman’s test was used for correlations. Correlation analysis was used to evaluate the relationship between vitamin D levels and cognitive performance of the patients with SLE. The level of one tailed p<0.05 was considered the cut-off value for significance.
Results
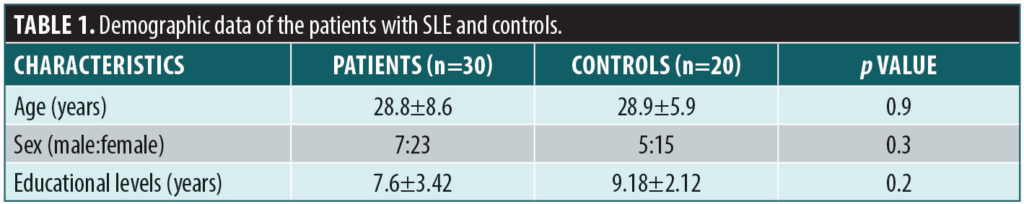
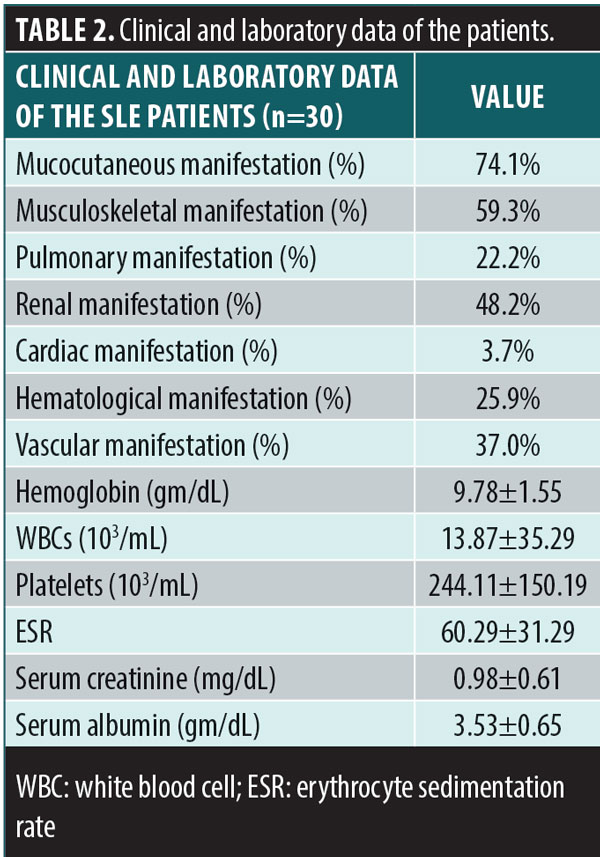
The demographic data of the patients and controls are itemized in Table 1. There was no significant difference in age, sex, or educational level between the two groups. The mean duration of the illness (months) among the SLE group was 32.33±29.96. The clinical and laboratory data of the SLE group are shown in Table 2.
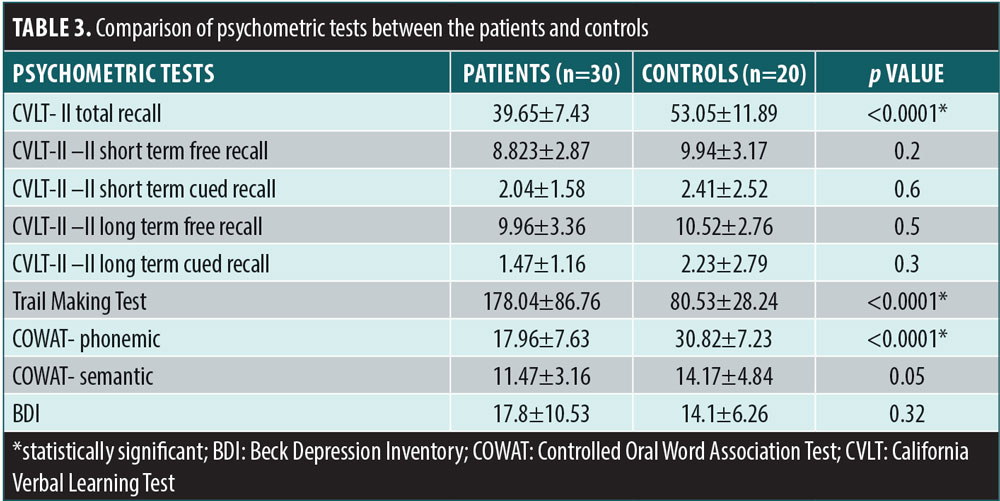
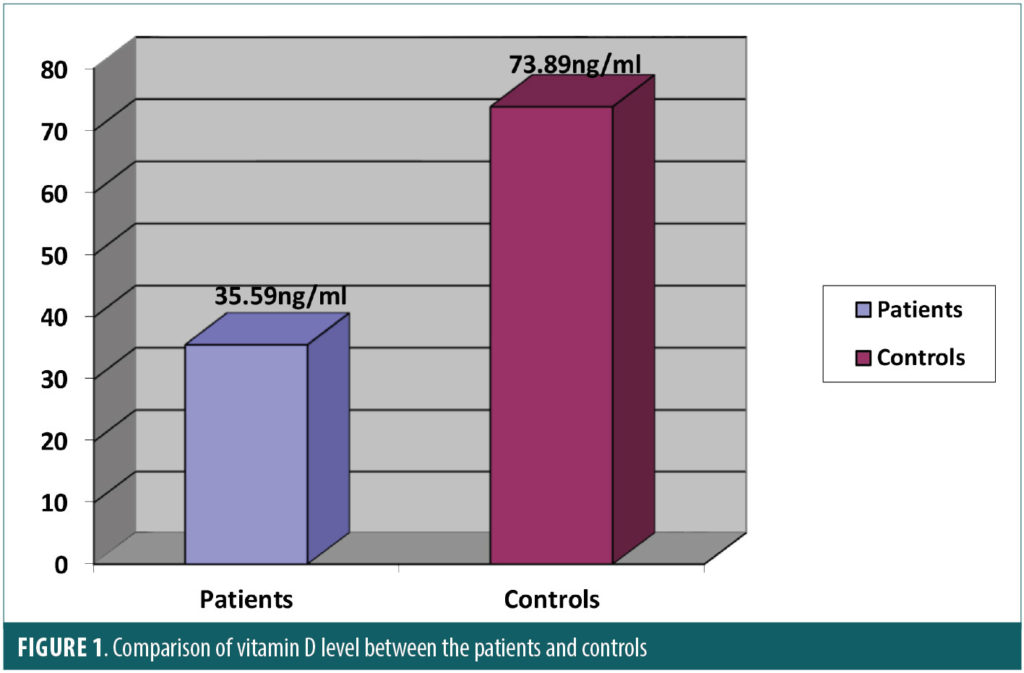
The comparison of the psychometric tests between the two groups is itemized in Table 3. Patients with SLE performed worse than controls in verbal memory total recall, executive function, and phonemic verbal fluency. There was a statistical significance between the two groups in CVLT-II total recall (p<0.0001), Trail Making Test (p<0.0001), and phonemic-COWAT(p<0.0001) scores. There was no a significant difference between the two groups in BDI scores (p=0.32). The mean of the 25-hydroxy vitamin D levels was 35.59±27.52ng/mL in the SLE group and 73.89± 15.24ng/mL in the control group. Subjects in the SLE group had significantly lower levels of vitamin D compared with the control group (p<0.0001) as shown in Figure 1.
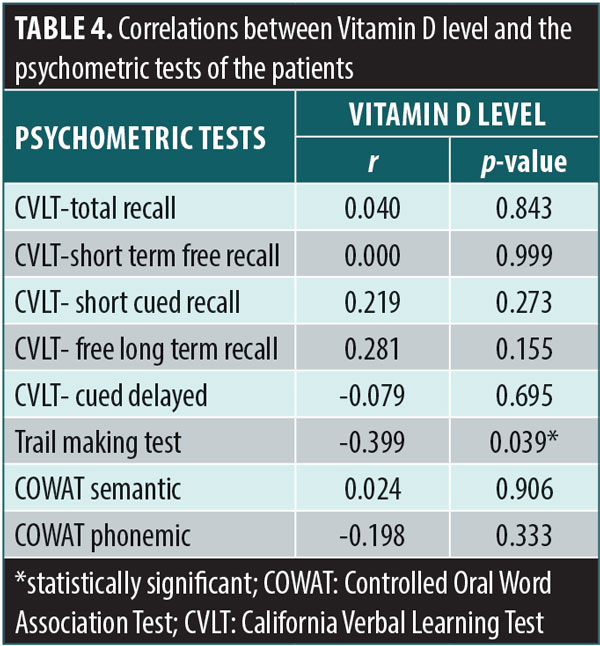
The correlation between the vitamin D levels and results of the different cognitive tests in the SLE group is shown in Table 4. There was a significant negative correlation between vitamin D levels and executive function as assessed by the Trail Making Test (r=-0.399, p=0.03). However, there was no a significant correlation between vitamin D levels and the other cognitive tests.
Discussion
The reported effects of vitamin D on cognitive function have primarily been shown in animal studies. Some studies suggest that low levels of serum 25(OH) D are associated with poor cognitive functions,24 whereas others report no such association.25,26
Cognitive dysfunction has been shown to have a significant impact on employment, social activities, and health-related quality of life in people with SLE,27 and has been reported in 20 to 80 percent of this patient poputlation.4,28 This wide range of variability might be due to multiple factors, including heterogeneity of patients and use of different assessment methods. In our study, we primarily focused on the detailed evaluation of cognitive patterns among patients with NPSLE rather than global cognitive assessment, particularly early in the course of the disease. To our knowledge, this detailed cognitive pattern evaluation and its association with vitamin D deficiency has not been well-studied in patients with SLE.
In the current study, we found that the patients with SLE performed significantly worse on executive function and verbal fluency testing, assessed by the Trail Making Test and COWAT, respectively, than the healthy controls. These results are similar to results from previous studies.28–31 Leritz et al31 reported that the most prevalent pattern of cognitive impairment in patients with SLE was the “subcortical” type in which executive functioning, information processing speed, and verbal fluency were frequently affected. These cognitive dysfunctions could be due to disruption of certain white matter neural circuits that connect subcortical structures and prefrontal cortex. Moreover, the subjects in the SLE group performed significantly more poorly on total recall of verbal memory and learning compared with the healthy controls. However, this is not common in the subcortical type of cognitive dysfunction. Hanly et al29 reported that patients with SLE exhibited more learning difficulties when compared with patients with rheumatoid arthritis, which could be attributed to the dysfunction of encoding material and suggests the possibility of memory impairment. It was noted that the patients experienced greater difficulty with acquisition than they did with retrieval.
In our study, the patients with SLE had significantly lower vitamin D levels than the healthy controls. Research suggests that vitamin D has a substantial role in activation of innate immunity, which subsequently results in regulation of acquired immunity. A relationship between vitamin D deficiency and some autoimmune diseases, such as SLE, rheumatoid arthritis, and insulin-dependent diabetes mellitus, has been documented.32
Patients with SLE have multiple risk factors for vitamin D deficiency. These patients usually suffer from photosensitivity and are advised to apply sunscreen, which can result in lower sun exposure and subsequently decreased production of vitamin D in the skin. Chronic treatment with corticosteroids and hydroxychloroquine can also influence vitamin D metabolism. In addition, severe renal involvement, which frequently presents in patients with lupus nephritis, can affect the hydroxylation step of 25(OH)D.33
In our study, there was a significant correlation between vitamin D and reduced executive functioning. Our results are in agreement with Tay et al,28 who found vitamin D to be a risk factor for cognitive impairment among patients with SLE. The 25(OH) D3 crosses the blood-brain barrier to reach vitamin D receptors (VDRs), which are present on neurons and glial cells of the CNS, often co-localized in cells expressing 1?-hydroxylase. This is necessary for conversion of 25(OH) D3 to the biologically active 1,2 (OH) 2D3 in the CNS, which is in turn facilitates proper cognitive brain functioning.34,35
There are several possible explanations as to why vitamin D levels are associated with proper executive functioning. As mentioned previously, vitamin D (in addition to its receptors and 1,?-hydroxylase) enhances neuroprotection through its effects on proinflammatory cytokines, which contribute to cognitive decline and dementia.9 Moreover, the vitamin D appears to regulate the intra-neuronal calcium homeostasis via voltage-gated calcium channels and, as an antioxidant, shows neuroprotective effects against glutamate toxicity.36 The 1,25(OH)2D3 appears to inhibit the expression of major histocompatibility complex Class II proteins, as well as trigger inflammatory cells to apoptosis. Functionally, the substantial expression of VDRs in the cortex and hippocampus suggests that 25(OH)D3 has a potentially significant impact on cognition.28
The capabilities of sophisticated magnetic resonance imaging (MRI) techniques, such 18F-fluorodeoxyglucose (18FDG) positron emission tomography (PET) and diffusion tensor imaging, which detect metabolic and structural abnormalities, respectively, could assist in further understanding of the effect that low levels of vitamin D level might have on cognitive function among patients with SLE. Imaging has revealed an increase in 18FDG uptake in the white matter, which is indicative of inflammatory activity. This suggests that cognitive dysfunction in patients with SLE might be the result of white matter inflammation with subsequent white matter tract damage.37 Also, it has been shown that patients with low vitamin D levels have lower fractional anisotropy values in the frontal regions.38 Vitamin D deficiency has been associated with neuronal integrity disruption, mainly in frontal regions, which could result in memory and executive function impairment.38
In our study, there was no significant difference between the patients with SLE and healthy controls regarding depression, as assessed by BDI. Moreover, there was no statistically significant correlation between depression and other psychometric tests. These results were in agreement with Tay et al,28 who reported no association between cognitive dysfunction and depression in patients with SLE.
Limitations. The small number of patients and lack of correlation between the vitamin D levels and immunosuppressant therapies and their impact on cognitive performance were important limitations in this study.
Conclusion
Based on our results, patients with SLE who do not have NPSLE might exhibit cognitive dysfunction that selectively affects certain cognitive domains, such as executive function, verbal fluency, and verbal memory. Cognitive dysfunction among patients with SLE might be attributed, at least in part, to vitamin D deficiency. Vitamin D deficiency appears to worsen prognosis among patients with SLE, and has been reported to have a significant negative impact on cognitive function in the general population. Vitamin D deficiency, evident in our patients with SLE, has been linked to limited sun exposure, which is common among this patient population due to its triggering aspects for SLE. Thus, vitamin D supplementation might be considered a useful adjunctive therapy for patients with SLE as a preventive or mitigating measure against symptoms of cognitive impairment. Studies with larger patient samples are needed to provide more robust results. Additionally, studies to determine the effects that corticosteroids and the other immunosuppressant drugs have on vitamin D levels in patients with SLE are needed.38
References
- Jeltsch-David H, Muller S. Neuropsychiatric systemic lupus erythematosus: pathogenesis and biomarkers. Nat Rev Neurol. 2014;10(10):
579–596. - Harrison MJ, Ravdin LD. Cognitive dysfunction in neuropsychiatric systemic lupus erythematosus. Curr Opin Rheumatol. 2002;14(5):510–514.
- Tay SH, Mak A. Anti-NR2A/B antibodies and other major molecular mechanisms in the pathogenesis of cognitive dysfunction in systemic lupus erythematosus. Int J Mol Sci. 2015;16(5):
10281–10300. - The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608.
- Zhao G, Ford ES, Li C, Croft JB. Serum 25-hydroxyvitamin D levels and all-cause and cardiovascular disease mortality among US adults with hypertension. J Hypertens. 2012;30(2):
284–289. - Littlejohns TJ, Henley WE, Lang IA, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83(10):920-928.
- Jelinek PL, Simpson S Jr, Brown CR, et al. Self-reported cognitive function in a large international cohort of people with multiple sclerosis: associations with lifestyle and other factors. Eur J Neurol. 2018 Aug 22. doi: 10.1111/ene.13784. [Epub ahead of print].
- Landfield PW, Cadwallader-Neal L. Long-term treatment with calcitriol (1,25(OH)2 vit D3) retards a biomarker of hippocampal aging in rats. Neurobiol Aging. 1998;19(5):469–477.
- Pettersen JA. Vitamin D and executive functioning: Are higher levels better? J Clin Exp Neuropsychol. 2016;38(4):467–477.
- Kamen DL, Aranow C. The link between vitamin D deficiency and systemic lupus erythematosus. Curr Rheumatol Rep. 2008;10(4):273–280.
- Balion C, Griffith LE, Strifler L, et al. Vitamin D, cognition, and dementia: A systematic review and meta-analysis. Neurology. 2012;79(13):
1397–1405. - Etgen T, Sander D, Bickel H, et al. Vitamin D deficiency, cognitive impairment and dementia: a systematic review and meta-analysis. Dement Geriatr Cogn Disord. 2012;33(5):297–305.
- Yap KS, Morand EF. Vitamin D and systemic lupus erythematosus: continued evolution. Int J Rheum Dis. 2015;18(2):242–249.
- Magro-Checa C, Zirkzee EJ, Huizinga TW, et al. Management of neuropsychiatric systemic lupus erythematosus: current approaches and future perspectives. Drugs. 2016;76:459–483.
- Hochberg MC. Updating the American college of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725–1725.
- Kozora E, Ellison MC, West S. Reliability and validity of the proposed American College of Rheumatology neuropsychological battery for systemic lupus erythematosus. Arthritis Rheum. 2004;51(5):810–818.
- Woods SP, Delis DC, Scott JC, et al. The California Verbal Learning Test—second edition: Test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch Clin Neuropsychol. 2006;21(5):413–420.
- Hegazy M, Kishk N, Shalaby N, et al. Reliability of BICAMS (Arabic version) in Egyptian. Multiple ECTRIMS Online Library. Kishk N. Oct 26 2017; 200048. ECTRIMS Online Library.
- Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–338.
- Abdel Aziz K, Khater MS, Emara T, et al. Effects of age, education, and gender on verbal fluency in healthy adult Arabic-speakers in Egypt. Appl Neuropsychol Adult. 2017;24(4):331–341.
- Corrigan JD, Hinkeldey NS. Relationships between parts A and B of the Trail Making Test. J Clin Psychol. 1987;43(4):402–409.
- Stanczak DE, Stanczak EM, Awadalla AW. Development and initial validation of an Arabic version of the Expanded Trail Making Test: implications for cross-cultural assessment. Arch Clin Neuropsychol. 2001;16(2):141–149.
- Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571.
- Sato Y, Honda Y, Hayashida N, et al. Vitamin K deficiency and osteopenia in elderly women with Alzheimer’s disease. Arch Phys Med Rehabil. 2005;86(3):576–581.
- Aung K, Burnett J, Smith SM, Dyer CB. Vitamin D deficiency associated with self-neglect in the elderly. J Elder Abuse Negl. 2006;18(4):63–78.
- Etgen T, Sander D, Bickel H, et al. Vitamin D deficiency, cognitive impairment and dementia: a systematic review and meta-analysis. Dement Geriatr Cogn Disord. 2012;33(5):297–305.
- Panopalis P, Julian L, Yazdany J, et al. Impact of memory impairment on employment status in persons with systemic lupus erythematosus. Arthritis Rheum. 2007;57(8):1453–1460.
- Tay SH, Ho CS, Ho RC-M, Mak A. 25-Hydroxyvitamin D3 deficiency independently predicts cognitive impairment in patients with systemic lupus erythematosus. PLoS One. 2015;10(12):e0144149.
- Hanly JG, Omisade A, Su L, et al. Assessment of cognitive function in systemic lupus erythematosus, rheumatoid arthritis, and multiple sclerosis by computerized neuropsychological tests. 2010;62(5):1478–1486.
- Waterloo K, Omdal R, Sjöholm H, et al. Neuropsychological dysfunction in systemic lupus erythematosus is not associated with changes in cerebral blood flow. J Neurol. 2001;248(7):
595–602. - Leritz E, Brandt J, Minor M, et al. Neuropsychological functioning and its relationship to antiphospholipid antibodies in patients with systemic lupus erythematosus. J Clin Exp Neuropsychol. 2002;24(4):527–533.
- Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood). 2004;229(11):1136–1142.
- Ruiz-Irastorza G, Egurbide M V, Olivares N, et al. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology. 2008;47(6):
920–923. - McGrath J, Feron F, Eyles D, Mackay-Sim A. Vitamin D: the neglected neurosteroid? Trends Neurosci. 2001;24(10):570–572.
- Johansson P, Almqvist EG, Johansson J-O, et al. Cerebrospinal fluid (CSF) 25-hydroxyvitamin D concentration and CSF acetylcholinesterase activity are reduced in patients with Alzheimer’s disease. PLoS One. 2013;8(11):e81989.
- Annweiler C, Schott A-M, Berrut G, et al. Vitamin D and ageing: neurological issues. neuropsychobiology. 2010;62(3):139–150.
- Ramage AE, Fox PT, Brey RL, et al. Neuroimaging evidence of white matter inflammation in newly diagnosed systemic lupus erythematosus. Arthritis Rheum. 2011;63(10):3048–3057.
- Moon Y, Moon W-J, Kwon H, et al. Vitamin D deficiency disrupts neuronal integrity in cognitively impaired patients. J Alzheimers Dis. 2015;45(4):1089–1096.





