
by Waguih William IsHak, MD; Ashley Meyer, BS; Luiza Freire, MD; Jayant Totlani, DO; Nathalie Murphy, MD; Sabrina Renteria, MD; Mohamed Salem, BS; Tiffany Chang, MS; Rasha Abdelsalam MD; Rida Khan, MD; Thomas Chandy, MS, DO; Thomas Parrish, BS; Drew Hirsch, BA candidate; Bhumika Patel, DO candidate;
Alexander J. Steiner, PsyD; Sarah Kim, MD; Rebecca Hedrick, MD;
Robert N. Pechnick, PhD; and Itai Danovitch, MD
Drs. IsHak, Murphy, Renteria, Abdelsalam, Khan, Kim, Hedrick, and Danovitch; Mr. Salem; Ms. Chang; Mr. Parrish; and Mr. Hirsch are with Cedars-Sinai Health System in Los Angeles, California. Dr. IsHak is also with David Geffen School of Medicine at UCLA in Los Angeles, California. Ms. Meyer is with University of California Irvine, School of Medicine in Irvine, California. Dr. Freire is with Faculdade Pernambucana de Saude in Recife, PE, Brazil. Drs. Totlani and Pechnick and Ms. Patel are with Western University of Health Sciences in Pomona, California. Dr. Chandy is with Loma Linda University in Loma Lina, California. Dr. Steiner is with Executive Mental Health, Inc. in Los Angeles, California.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Innov Clin Neurosci. 2024;21(7–9):27–47.
Abstract
Objective: This systematic review provides an overview of psychiatric medications in the late stages of development (Phase III clinical trials) as of June 1, 2024. It details the mechanisms of action, efficacy, dosing, and adverse effects of these medications.
Methods: We searched the PubMed database for Phase III studies of psychiatric medications published until June 1, 2024, using the keywords “psychiatric” OR “psychopharm*” AND “medic*” OR “pharm*”. Our review encompassed medications currently undergoing Phase III clinical trials and those that have completed Phase III but are awaiting approval from the United States Food and Drug Administration (FDA). We independently analyzed the identified studies and reached a consensus on the medications to be included in this systematic review.
Results: As of June 1, 2024, a total of 89 pipeline drug trials were identified, including nine for schizophrenia, five for bipolar disorders, 25 for depressive disorders, 11 for anxiety disorders, five for post-traumatic stress disorder (PTSD), one for obsessive compulsive disorder (OCD), two for eating disorders, two for sleep-wake disorders, three for sexual dysfunctions, one for substance-related and addictive disorders, 22 for neurocognitive disorders, and three for neurodevelopmental disorders, specifically attention deficit hyperactivity disorder (ADHD).
Conclusion: The psychiatric medications in the pipeline as of June 1, 2024, demonstrate significant promise in treating psychiatric disorders.
Keywords: Psychiatric medications, Phase III clinical trials, schizophrenia, bipolar disorder, depression, anxiety, psychiatric disorders, FDA
Psychiatric medications undergoing United States Food and Drug Administration (FDA) evaluation progress through three distinct clinical trial phases. Phase I aims to establish safety profiles and determine appropriate dosages and typically involves 20 to 100 healthy volunteers or individuals with the target disease/condition over a study duration of several months. Phase II assesses efficacy and side effects and typically extends to several hundred participants with the target condition over several months to years. Phase III determines efficacy and safety in a larger population, typically 300 to 3,000 participants diagnosed with the condition, over 1 to 4 years.1 The purpose of this systematic review is to examine the psychiatric medications in Phase III clinical trials up to June 1, 2024, delineating their mechanisms of action, evidence of efficacy, dosing protocols, and adverse effects. Our goal is to offer clinicians a comprehensive and user-friendly review of these emerging psychiatric medications, equipping them with valuable insights to prepare for potential new treatment options upon regulatory approval.
Methods
Studies of Phase III psychiatric medications published until June 1, 2024, were identified from the PubMed database using the keywords: “psychiatric” OR “psychopharm*” AND “medic*” OR “pharm*”. The authors independently conducted a focused analysis and reached a consensus on the medications to include in this systematic review. Key findings were extracted and summarized from the full text and tables of the selected studies.
Results
Overview. Psychiatric medications undergoing development in Phase III trials were fully described. Medications currently in Phase III studies and those that have completed Phase III and are awaiting FDA approval were included (Table 1). We organized the list of psychiatric medications by psychiatric disorder according to the nomenclature of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). An overview of medications for each psychiatric disorder grouped by characteristics and indications is accompanied by an alphabetically organized summary table containing the medication’s generic, as well as other, names, mechanism of action, indication(s) being tested in Phase III, route and dosage, and notes for clinicians, including effects on sedation, weight/lipids, extrapyramidal tract, prolactin, sexual dysfunction, and QTc.
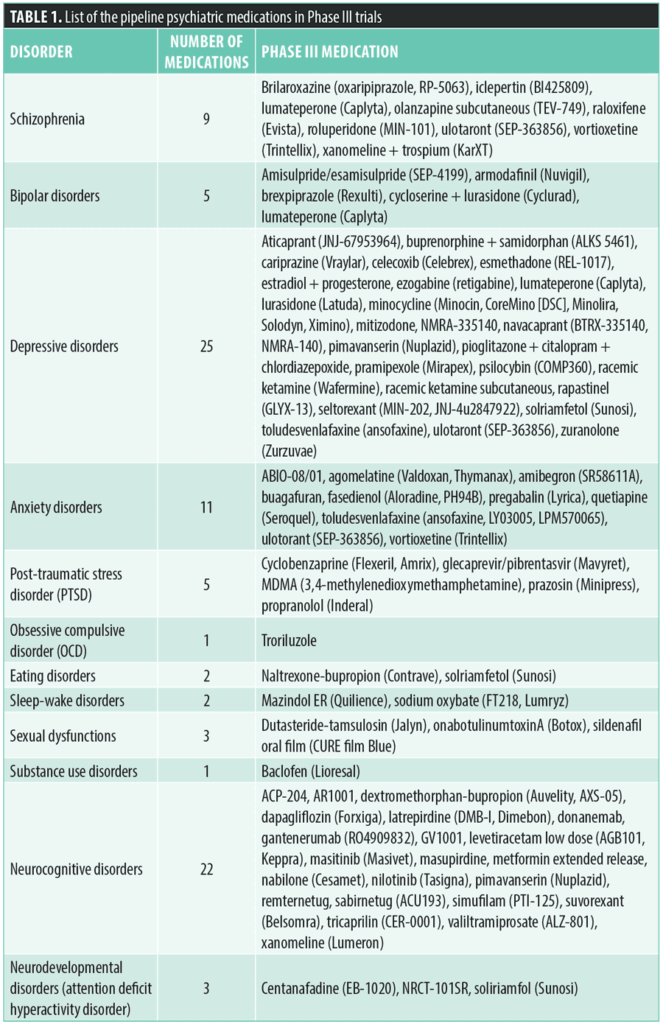
Schizophrenia. Recent Phase III trials have showcased promising investigational medications targeting cognitive and psychotic symptoms in schizophrenia, each with distinct mechanisms of action and pharmacological profiles.
Brilaroxazine (oxaripiprazole, RP-5063), administered at dosages ranging from 5 to 100mg daily, acts as a partial agonist at dopamine D2, D3, D4, and serotonin receptors (5-HT1A, 5-HT2A, 5-HT2B), also blocking 5-HT6 and 5-HT7 receptors. It is being studied for its potential to treat both cognitive and psychotic symptoms, showing minimal impact on weight, lipids, prolactin levels, blood pressure, or electrocardiogram (EKG). The most commonly reported side effects include somnolence and akathisia.2,3
Iclepertin (BI-425809), a potent glycine transporter 1 (GlyT1) inhibitor, has demonstrated safety and good tolerance in both healthy volunteers and patients with schizophrenia. By inhibiting GlyT1, iclepertin raises glycine levels in the cerebrospinal fluid, thereby enhancing N-methyl-D-aspartate (NMDA) receptor activity and improving glutamatergic signaling. This is crucial for neural synchrony and synaptic plasticity, which are often impaired in schizophrenia due to NMDA receptor hypofunction.4,5 Early studies indicate that it improves memory performance and electroencephalogram (EEG) parameters, with clinical trials showing significant cognitive improvements at daily doses of 10mg and 25mg, and it was well tolerated at doses up to 75mg. Phase III trials are now assessing its long-term effects on daily functioning.3,6
Xanomeline plus trospium (KarXT) is a promising treatment for schizophrenia, combining the effects of xanomeline and trospium to target psychotic symptoms effectively while minimizing common side effects. Xanomeline, an M1/M4 muscarinic agonist, can readily cross the blood-brain barrier (BBB) and stimulate muscarinic receptors in the brain, addressing both negative and potentially positive symptoms of schizophrenia. Trospium, a peripheral and nonselective muscarinic antagonist, cannot cross the BBB and thus acts primarily outside the brain. By blocking peripheral muscarinic receptors, trospium reduces side effects that xanomeline might cause in other organs. Clinical trials have demonstrated that KarXT significantly improves outcomes in patients with schizophrenia, including marked improvements in the Positive and Negative Syndrome Scale (PANSS) total score and cognitive function, compared to placebo. Additionally, KarXT enhances working memory and linguistic cognition without causing sedation, weight gain, or extrapyramidal side effects often associated with other antipsychotic medications.7,8
Ulotaront (SEP-363856), a trace amine-associated receptor 1 (TAAR1) and serotonin 5-HT1A receptor agonist administered in 25 to 75mg daily doses, has been noted for its lack of extrapyramidal side effects and minimal impact on weight and metabolism, maintaining stable prolactin levels.9–12 Over 92 percent of the drug is excreted in urine, with no clinically meaningful drug interactions involving ulotaront or its metabolites and cytochrome P450 (CYP) enzymes or transporters.13
Lumateperone is already FDA-approved for treating adult schizophrenia and bipolar disorder, either as monotherapy or adjunctive therapy with lithium or valproate. Currently, it is under investigation for pediatric use. This antipsychotic targets dopaminergic, serotonergic, and glutamatergic pathways. It acts as a presynaptic partial agonist and postsynaptic antagonist at dopamine D2 receptors, reducing dopamine in the synaptic cleft while blocking postsynaptic D2 receptors. It requires only 40-percent D2 receptor occupancy, which results in a lower risk of extrapyramidal side effects. Lumateperone also antagonizes 5-HT2A receptors and augments NMDA and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor activity in the prefrontal cortex, contributing to its antipsychotic and antidepressant effects. Clinical trials have shown its efficacy in treating acute schizophrenia with minimal metabolic side effects and potential benefits for negative symptoms. For pediatric use, it is usually taken at 42mg daily, with the most common side effects being dizziness, nausea, and dry mouth.14–16
The extended-release subcutaneous formulation of olanzapine (TEV-749) is currently being studied as a potential treatment for schizophrenia. This medication acts as an antagonist for dopamine D2 and serotonin 5-HT2A and is administered via subcutaneous injection once a month. The study is comparing three different doses, but the specific milligram amounts have not been disclosed yet. Safety and side effect data for this treatment are still awaiting publication.17
Raloxifene, an estrogen receptor modulator, is under investigation for its potential to alleviate negative symptoms in postmenopausal women with schizophrenia. Studies have indicated cognitive benefits, particularly in working memory, with a daily oral dose of 120mg. However, weight gain has been noted as a potential side effect of this treatment.18,19
Roluperidone (MIN-101) acts on serotonin 5-HT2A and alpha-1A adrenergic receptors and is known for its lack of extrapyramidal side effects and its negligible impact on weight, metabolic function, and prolactin levels. Administered at doses of 32 to 64mg per day, this medication has exhibited significant efficacy in addressing negative symptoms in schizophrenia.20,21
Vortioxetine, a 5-HT multimodal agent, has been approved for major depressive disorder (MDD) since 2013. It is being investigated for cognitive enhancement and action on negative symptoms in patients with schizophrenia at doses of 5 to 20mg daily. Animal studies have shown it improves cognitive and social behaviors, and clinical studies indicate it improves social and physical anhedonia, particularly when combined with olanzapine. Adverse effects include nausea, constipation, potential mania activation, and serotonin syndrome.22–27
This condensed overview provides a grouped perspective on investigational medications, offering insight into their shared characteristics and potential applications in schizophrenia. For further details, Table 2 presents comprehensive information on each medication.
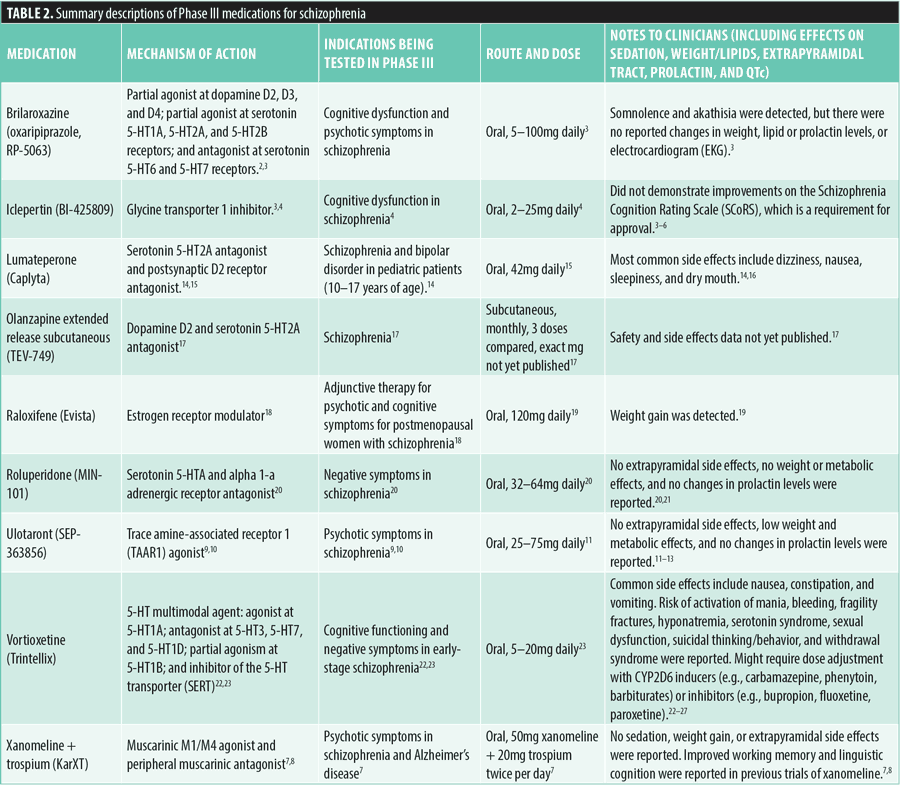
Bipolar disorders. Pipeline medications for the treatment of bipolar disorders were found to have both traditional and novel mechanisms.
Amisulpride plus esamisulpride (SEP-4199) combines a dopamine D2 and serotonin 5-HT7 antagonism to target bipolar I depression. Administered orally in dosages ranging from 200 to 400mg daily, clinical trials report somnolence and extrapyramidal effects, such as akathisia, minimal changes in weight and lipids, elevated prolactin levels, and QTc prolongation.28
Armodafinil (Nuvigil), a sympathomimetic and dopamine reuptake inhibitor, is also being studied as an adjunctive therapy for depressive symptoms in bipolar disorder with a daily dose of 150mg. The medication is generally well tolerated, with common side effects being headache and nausea.29,30
Brexpiprazole (Rexulti) is being investigated for bipolar I depression at dosages of 2 to 4mg daily. It is a partial agonist at D2 and 5-HT1A receptors and an antagonist at 5-HT2A receptors. This medication offers potential benefits with reduced risks of extrapyramidal symptoms and hyperprolactinemia, compared to other antipsychotics. Clinical trials have shown its effectiveness in reducing depressive symptoms in bipolar I disorder, with potential side effects including weight gain, somnolence, and akathisia. Brexpiprazole has a long half-life and stable pharmacokinetics, making it easier for patients to adhere to the treatment regimen. The drug is metabolized by CYP2D6 and CYP3A4, necessitating monitoring for potential interactions.14,31
The combination of cycloserine plus lurasidone (NRX-101, Cyclurad), acting as an antagonist at the glycine coreceptor of the NMDA receptor plus antagonist at D2, 5-HT2A, and 5-HT7 receptors, is being explored for the maintenance of remission in bipolar depression with suicidal ideation. Administered at a daily combined dose of 950mg (D-cycloserine) and 66mg (lurasidone), it has shown low-to-moderate sedation, elevated prolactin levels, and extrapyramidal effects, with minimal impact on weight and lipids and no clinically relevant QTc prolongation.32
Lastly, lumateperone is being investigated for its effectiveness in treating major depressive episodes associated with bipolar I or II disorder in pediatric patients aged 10 to 17 years. It is taken orally at a dosage of 42mg daily and generally causes side effects such as dizziness, nausea, sleepiness, and dry mouth.14,33
For further details on each medication, refer to Table 3.
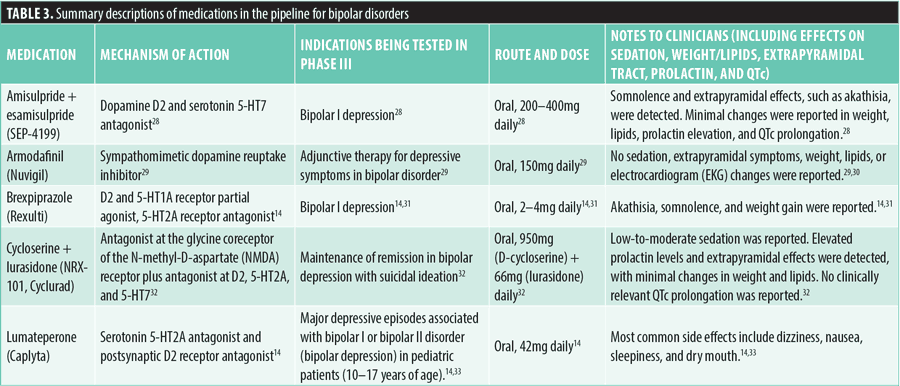
Depressive disorders. As of June 1, 2024, 25 medications were in the pipeline for approval for the treatment of depressive disorders and their associated symptoms. Many of the medications reviewed showed similar mechanisms of action, notably 5-HT serotonin agonism and antagonism and kappa-opioid receptor antagonism. However, several medications introduce novel or distinct mechanisms of action.
Adjunctive therapy involving a combination of citalopram, chlordiazepoxide, and pioglitazone (used for Type 2 diabetes) has been evaluated for significant depression treatment at a daily dose of 15mg. However, the tolerability and safety of this combination remains a concern. This medication combines a peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist (pioglitazone) with a selective serotonin reuptake inhibitor (citalopram) and a benzodiazepine (chlordiazepoxide). Pioglitazone might cause weight gain in patients with diabetes and increase the risk of heart failure and edema, while chlordiazepoxide has shown similar effects in animal models.34–37
Medications such as rapastinel, seltorexant, toludesvenlafaxine, psilocybin, racemic ketamine, and zuranolone introduce distinct mechanisms of action.
Rapastinel (GLYX-13) is an NMDA receptor modulator with partial agonist properties at the glycine site. It is administered intravenously at doses of 225mg, 450mg, or 900mg on a weekly or biweekly basis. Early trials demonstrated rapid and sustained antidepressant effects in individuals with treatment-resistant depression (TRD) without causing psychotomimetic effects. However, it failed to meet primary or secondary endpoints in subsequent Phase III trials. Ongoing studies are evaluating its long-term antidepressant effects in individuals with MDD, both as an additional treatment and as a standalone therapy. The drug has been shown to have no sedative effects or significant impact on QTc interval, and in animal studies, it has exhibited the potential to alleviate memory deficits.38–41
Seltorexant (MIN-202, JNJ-42847922) antagonizes orexin-2 receptors, which regulate wakefulness and arousal. It is being investigated for the treatment of MDD and insomnia with daily doses of 10 to 40mg. Common adverse events included somnolence, headache, and nausea, with some participants experiencing elevated liver enzymes and sleep issues, leading to discontinuation.42,43
Toludesvenlafaxine (ansofaxine), a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI), is being evaluated for MDD at oral doses ranging from 40 to 160mg daily. Past studies have shown potential impacts on liver function with elevated bilirubin and alanine transferase levels.44,45
Psilocybin, a 5-HT1A/5-HT2A receptor agonist, is presently under investigation for MDD. The compound is typically administered orally in doses ranging from 20 to 30mg. Notably, studies have demonstrated the efficacy of psilocybin in reducing weight gain and altering the trajectory of obesity in animal models.46–48
Two formulations of racemic ketamine, an NMDA receptor antagonist, are currently under study for the treatment of MDD and TRD: Wafermine, a sublingual racemic ketamine, and subcutaneous racemic ketamine. Wafermine is administered sublingually at doses of 300 to 450mg/kg at various frequencies. It shows promise in treating MDD but is associated with elevated blood pressure and the potential to induce hypertension.49 Subcutaneous racemic ketamine is administered at doses of 0.5 to 1mg/kg for TRD. Possible side effects might include hallucinations, dissociation, sedation, disorientation, and insomnia.50
Pramipexole, a full dopamine agonist FDA-approved for Parkinson’s disease and restless legs syndrome, demonstrates marked selectivity for D3 receptors. It is implicated in mood regulation and has shown neuroprotective and anti-inflammatory properties, suggesting utility in depression treatment, specifically for anhedonia.51,52 It is taken orally at 0.125mg twice daily and might cause side effects, such as nausea, dizziness, headache, fatigue, insomnia, constipation, hallucinations, orthostatic hypotension, impulse control disorders, and daytime sleepiness.51–54
Kappa opioid receptors are targeted by aticaprant and navacaprant, acting as antagonists. Aticaprant (JNJ-67953964) is being studied as an adjunctive therapy for MDD with moderate-to-severe anhedonia. It is taken orally at a 10mg daily dose and has no significant effects on sedation, sexual dysfunction, weight gain, or QTc interval activity.55,56 Navacaprant (BTRX-335140, NMRA-140) is under investigation as a monotherapy for MDD, administered orally at 80mg daily. In Phase II trials for mild-to-moderate MDD, significant improvements were noted at four weeks, but not sustained at eight weeks, compared to placebo. This compound also improved working memory and executive functioning in animal models.57
Buprenorphine plus samidorphan (ALKS 5461) combines a kappa opioid receptor agonist with a mu opioid receptor partial agonist. It is being explored for TRD, taken sublingually in doses ranging from 4mg plus 4mg to 8mg plus 8mg daily. Previous studies on opioid dependence treatment suggest possible adverse effects, including sexual dysfunction and premature ejaculation in more than 80 percent of participants.58,59
Cariprazine and lurasidone offer promising results in monotherapy for MDD through antagonism at D2 and 5-HT receptors. Cariprazine (Vraylar) is being studied at daily doses of 1 to 4.5mg. It is a partial agonist at central D2 and 5-HT1A receptors, with antagonist activity at the 5-HT2A receptor. It is already FDA-approved for schizophrenia and bipolar disorder, and it was also approved in December 2022 as adjunctive therapy for MDD.14,60 Lurasidone (Latuda), which antagonizes 5-HT2A/D2 receptors with additional 5-HT7 antagonism, is being tested at doses ranging from 20 to 60mg daily, with infrequent mild sedation reported.14,32
Solriamfetol and ulotaront both target MDD by acting as agonists at TAAR1. Solriamfetol (Sunosi), a dopamine and norepinephrine reuptake inhibitor and TAAR1 agonist, is being tested at an oral dose of 300mg for MDD. It is approved for managing excessive daytime sleepiness due to obstructive sleep apnea (OSA) or narcolepsy, with common side effects including headache, nausea, and decreased appetite.61,62 Ulotaront (SEP-363856), a TAAR1 agonist with additional agonism at 5-HT1A, has been studied for treating psychotic symptoms in schizophrenia and as an adjunctive therapy in MDD, with a daily dose of 25 to 75mg and no significant side effects.10–12
Several novel therapies are under investigation, primarily focusing on modulating the serotonin system. Pimavanserin (Nuplazid), an inverse agonist at serotonin 5-HT2A and 5-HT2C receptors, is being studied for MDD, dementia-related psychotic symptoms, and residual psychotic symptoms in schizophrenia. The recommended daily dose is 20mg. It is already FDA-approved for Parkinson’s disease-related psychosis and might affect QTc interval.63–67 Lumateperone tosylate (Caplyta) is a 5-HT2A antagonist being investigated for use in MDD. Administered at 42mg daily, it has a low risk of sedation, extrapyramidal side effects, weight gain, prolactin changes, and QTc prolongation.14,33,68 Mitizodone is a 5-HT receptor antagonist and a 5-HT1A receptor partial agonist being investigated as a monotherapy for MDD and is administered orally at doses ranging from 10 to 40mg daily. Additional research is needed to fully assess the adverse effects of this medication.69
Esmethadone, estradiol plus progesterone, ezogabine, and zuranolone represent innovative approaches targeting complex symptoms of depression, including treatment resistance and anhedonia, through unique mechanisms of action.
Esmethadone, an NMDA receptor antagonist, is being studied for TRD with a recommended daily oral dose of 25 to 50mg. Its predecessor, methadone, was known to contribute to weight gain, so further observation of esmethadone for similar effects is warranted.70,71
Estradiol plus progesterone, acting as an estrogen receptor agonist and progesterone receptor agonist, is being investigated for the prevention of depressive symptoms in peri-and postmenopausal female patients. It is administered orally with a dosage of 0.45mg of estrogen and 200mg of progesterone daily. Reported side effects include nausea, changes in bleeding levels, musculoskeletal pain, weight gain, and headaches.72,73
Ezogabine (retigabine), a positive allosteric modulator of KCNQ2 and KCNQ3 potassium channels, is being assessed for anhedonia and other depressive symptoms, with an oral dosing regimen titrated up to 900mg/day. Previously approved for focal onset seizures, ezogabine use has been associated with dizziness, confusion, and headaches.74 Zuranolone, a GABAA receptor agonist, is being tested as an adjunctive therapy for MDD at a daily oral dose of 30 to 50mg. It was recently FDA-approved for postpartum depression. Sedation was a frequent side effect in over five percent of participants in clinical trials.75
Extensive studies confirm the proinflammatory status in depression, with causal links to neurotransmitter dysregulation.76 Some clinical studies have demonstrated the positive benefits of celecoxib and minocycline in improving depressive symptoms. Celecoxib inhibits cyclooxygenase-2 (COX-2) and is currently being investigated as a potential monotherapy or adjunctive therapy for MDD. It is typically prescribed at a daily oral dose of 200 to 400mg and increases the risk of cardiovascular events and gastrointestinal bleeding, with potential renal and hepatic toxicity.77–81 Minocycline, a tetracycline antibiotic with antineuroinflammatory properties, is being studied as an adjunctive treatment for MDD at 200mg daily. It can penetrate the BBB, contributing to its neuroprotective potential. However, its use is associated with gastrointestinal effects, photosensitivity, and potentially severe autoimmune reactions.82,83
For more detailed information on each medication, refer to Table 4.
Anxiety disorders. As of June 1, 2024, there were 11 medications in the pipeline for approval in the treatment of anxiety disorders. While the majority are being investigated for generalized anxiety disorder (GAD), some also target symptoms associated with social anxiety disorder.
Several medications under investigation focus on modulating the serotonin system and GABA receptors. Pregabalin and fasedienol work on or modulate GABA receptors, while vortioxetine, quetiapine, and agomelatine act on serotonin (5-HT) family receptors, among others.
Agomelatine, a melatonin receptor agonist and serotonin 5-HT2C receptor antagonist, is being studied for the treatment and relapse prevention of GAD. Administered orally at doses of 25 to 50mg daily, it has shown no effects on weight gain, sexual dysfunction, or sedation, although a single case study observed prolonged QTc intervals.84,85
Pregabalin (Lyrica), a GABA analogue and voltage-gated calcium channel modulator, is being explored both as an adjunctive therapy and monotherapy for GAD at doses of 300 to 600mg daily. Side effects include sedation, weight gain, and potential sexual dysfunction, such as loss of libido, erectile dysfunction, and anorgasmia.86,87
Quetiapine extended release (Seroquel XR), a multimodal agent affecting histamine, dopamine, 5-HT, and norepinephrine receptors, is being evaluated for GAD. The typical dose ranges from 50 to 300mg daily. Significant sedation is noted, but it has less of an impact on weight, lipids, extrapyramidal symptoms, prolactin, and QTc intervals.88,89
Fasedienol (Aloradine, PH94B), which potentially acts on GABA receptors, is being assessed for acute anxiety in patients with social anxiety disorder. It is administered intranasally as needed, up to four doses daily, with side effects similar to placebo.90,91
Other medications, including ABIO-08/01, buagafuran, SR58611A (Amibegron), toludesvenlafaxine (ansofaxine), ulotaront (SEP-363856), and vortioxetine (Trintellix), focus on specific neurotransmitter pathways.
ABIO-08/01, which inhibits GABA and glutamate-gated chloride channels, is being tested for GAD symptoms. Administered orally at doses of 10 to 40mg daily, it has shown improvements in cognitive functioning and psychomotor activity without significant adverse effects.92
Buagafuran, which modulates central monoamine neurotransmitters and inhibits neuronal delayed rectifier potassium channels, is being studied for GAD treatment at doses of 30 to 120mg daily. Extensive CYP3A and CYP2E metabolism is noted, with dizziness being the most common side effect in early trials.93
SR58611A (Amibegron) introduces a distinct mechanism of action through selective beta-3 adrenoceptor agonism and is administered orally at doses of 0.3 to 10mg/kg daily for the treatment of GAD. Previous studies have reported no adverse effects on cognitive functioning, dependence, or alcohol interaction.94
Toludesvenlafaxine (ansofaxine), a first-in-class triple monoaminergic reuptake inhibitor (TRI) blocking the reuptake of serotonin, dopamine, and norepinephrine, is being evaluated for GAD at doses of 80 to 160mg daily. Common adverse events include nausea, vomiting, headache, and drowsiness. Sexual functioning should be monitored due to changes in prolactin and testosterone levels observed in preclinical studies.44,45
Ulotaront (SEP-363856), a TAAR1 and serotonin 5-HT1A receptor agonist, is being studied for GAD at doses of 25 to 75mg daily. Initial trials have reported somnolence and gastrointestinal symptoms, but no extrapyramidal side effects, changes in weight, metabolic effects, prolactin changes, or QTc interval prolongation were reported.10,95
Vortioxetine (Trintellix), a 5-HT multimodal agent, is being investigated for relapse prevention in GAD. It is administered orally at 10 to 20mg daily. Dose-dependent sexual dysfunction has been reported, ranging from 16 to 34 percent, compared to 14 to 20 percent with placebo.22,23,27,96
For more detailed information on each medication, refer to Table 5.
Post-traumatic stress disorder (PTSD). Five medications for PTSD were in the approval pipeline as of June 1, 2024, targeting various mechanisms to address symptoms of PTSD, including sleep disturbances and comorbid conditions, such as hepatitis C infection.
Cyclobenzaprine (TNX-102 SL) is an alpha-1 adrenergic, H1-histaminergic, M1-muscarinic, and 5-HT2A receptor antagonist being studied for its potential to address sleep disturbances related to PTSD, especially those affecting sleep-dependent memory consolidation. This substance is administered sublingually at doses of 5 to 6mg daily and is primarily recognized as a muscle relaxant for sudden-onset and acute muscle spasms. Clinical trials have reported sedative effects associated with its use.97
Prazosin, an alpha-1 adrenergic receptor antagonist, is under investigation for its efficacy in treating PTSD, especially nightmares and sleep disturbances, and PTSD associated with alcohol use disorder. It is administered orally at 1 to 20mg daily, and common side effects include dizziness, headache, drowsiness, lack of energy, weakness, palpitations, and nausea.98,99
Propranolol, a beta-adrenergic receptor blocker, is under investigation for the treatment of PTSD in children. It is taken orally at doses ranging from 20 to 120mg daily. Commonly reported adverse effects include bradycardia, sedation, thrombocytopenic purpura, and bronchospasm. Notably, a trial has observed sex differences in the reduction of PTSD symptoms, with male patients showing more reduction in symptoms compared to female patients.101
Glecaprevir/pibrentasvir is a combination of a NS3/4A protease inhibitor (glecaprevir) and a NS5A protein inhibitor (pibrentasvir), being tested for PTSD in patients infected with hepatitis C. Administered orally as three tablets once daily (glecaprevir 100mg + pibrentasvir 40mg), headache and fatigue are the primary noted side effects.102,103
3,4-methylenedioxy-methamphetamine (MDMA), known for its action as a releaser of serotonin, dopamine, and noradrenaline, is being evaluated for PTSD treatment. Administered orally at doses of 75 to 125mg daily, it was granted breakthrough therapy designation as assisted therapy for PTSD in 2017. Although risks of dependence, neurotoxicity, and cardiovascular toxicity have been described, recent PTSD trials have not reported these issues.104,105
For more details on these medications, refer to Table 6.
Obsessive compulsive disorder. As of June 1, 2024, only one medication has advanced to Phase III clinical trials for treating obsessive compulsive disorder (OCD). Troriluzole functions as a glutamate release inhibitor and a glutamate glial uptake stimulator taken orally at 200 to 280mg daily doses. Clinical trials have not reported significant concerns about sedation, weight gain, sexual dysfunction, or QTc interval changes, making it a promising treatment for OCD without notable adverse effects.106,107 See Table 7 for more details.

Feeding and eating disorders. Two medications currently in Phase III trials target binge eating disorder by utilizing different mechanisms of action.
Naltrexone plus bupropion is an FDA-approved medication for obesity. Early results show potential therapeutic use in treating binge eating disorder. It combines naltrexone, an opioid receptor blocker, and bupropion, a drug that affects norepinephrine and dopamine levels. The medication is taken orally in doses of naltrexone 16mg and bupropion 180mg daily. Common side effects include nausea, vomiting, constipation, dizziness, and headache. Serious side effects might include seizures, high blood pressure, and increased heart rate.108
Solriamfetol (Sunosi) is FDA-approved for excessive daytime sleepiness due to OSA or narcolepsy and is under Phase III investigation for binge eating disorder in adults. It is a dopamine and norepinephrine reuptake inhibitor and TAAR1 agonist, taken orally once per day at 150mg or 300mg doses. Common side effects include headache, nausea, and decreased appetite.109,110
For a comprehensive overview, please refer to Table 8.
Sleep-wake disorders. As of June 1, 2024, only two medications were identified in the pipeline for approval in the treatment of sleep-wake disorders.
Sodium oxybate (FT218, Lumryz), which acts as a GABAB receptor agonist, is under investigation for treating cataplexy and narcolepsy symptoms. It is taken orally at 6 to 9g nightly doses. However, it is under FDA review due to concerns about adverse effects, including the risk of dependence, leading to its classification as a Schedule III controlled substance with a restricted safety program for prescription.111,112
Quilience (Mazindol ER), a triple-reuptake inhibitor (SNDRI) and partial agonist for orexin (hypocretin)-II receptors, is also being studied for narcolepsy and comorbid cataplexy. Mazindol has shown improvements in narcolepsy symptoms when taken orally at a dose of 3mg nightly. However, it did not demonstrate improvements in cataplexy or sleep paralysis at this dose. At a higher dose of 6mg, it exhibited similar efficacy to dextroamphetamine 50mg for treating narcolepsy, with fewer adverse effects.113
For further details, refer to Table 9.
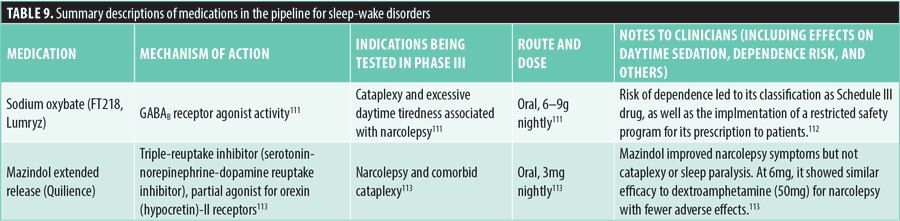
Sexual dysfunctions. Three medications are currently in Phase 3 trials for the treatment of sexual dysfunction, specifically erectile disorder. These medications utilize different mechanisms of action to address this condition.
Dutasteride-tamsulosin combines two active components: dutasteride, which blocks the conversion of testosterone to dihydrotestosterone (DHT), a key androgen involved in prostate gland development and growth, and tamsulosin, an alpha-blocker that relaxes the muscles in the bladder and prostate. This combination is administered orally at 0.5mg dutasteride and 0.4mg tamsulosin once daily. Common side effects include dizziness, abnormal ejaculation, decreased libido, and impotence.114,115
OnabotulinumtoxinA works by blocking the presynaptic release of the neurotransmitter acetylcholine at the neuromuscular junction. It is being tested for erectile disorder through intracavernous injections of 100 units once daily. Reported side effects include mild penile pain on injection and mild penile pain for three days following injection, with no systemic effects noted.116,117
Sildenafil oral film, a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase 5 (PDE5), is also under investigation for erectile disorder. This medication is taken orally at daily doses of 25 to 100mg. In trials involving healthy volunteers, a small number of patients experienced mild-to-moderate headaches and vomiting.118,119
For more detailed information about these medications, refer to Table 10.
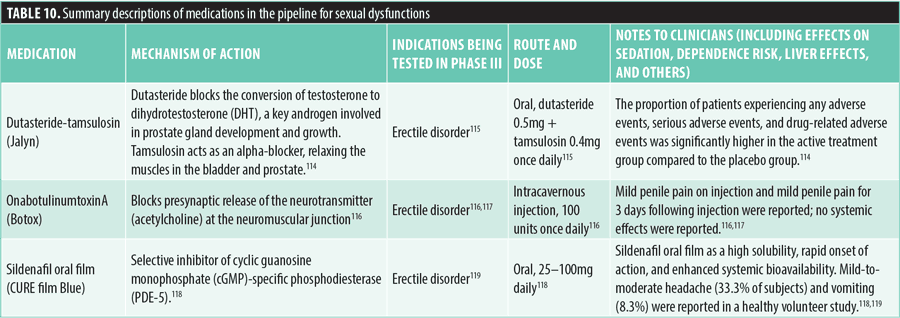
Substance use disorder. As of June 1, 2024, only one medication was in the approval pipeline for the treatment of substance use disorder. Baclofen (Lioresal), a GABAB receptor agonist, is being investigated for the treatment of alcohol use disorder. Administered orally at a dose of 30mg daily, baclofen is notable for its limited liver metabolism, which makes it a promising option for patients with liver damage. Clinical trials have reported somnolence as a common side effect. Previous studies have shown that in patients with kidney disease, adverse effects, such as delirium and symptoms of mania, might occur.120 Refer to Table 11 for additional details on baclofen.
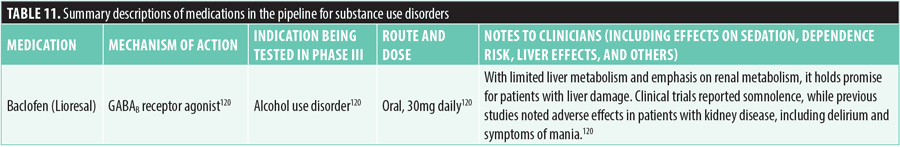
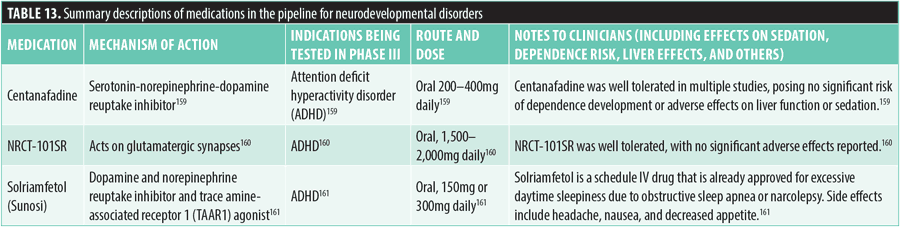
Neurocognitive disorders. Twenty-two new medications have been investigated for the treatment of neurocognitive disorders in Phase III trials. These medications are generally indicated for the treatment of Alzheimer’s disease and associated agitation and the clinical syndrome of dementia, which is typically operationally defined as a decline in cognition and functional abilities relative to an individual’s baseline level of function. However, such medications are diverse in their unique mechanisms of action.
A significant focus of current research involves targeting amyloid beta, a protein that accumulates abnormally in the brains of individuals with Alzheimer’s disease, contributing to neurodegeneration. These medications aim to reduce or neutralize amyloid beta to slow disease progression and alleviate symptoms.121
The monoclonal antibody sabirnetug (ACU193) targets soluble amyloid beta oligomers implicated in Alzheimer’s disease progression and neurodegeneration. This medication is delivered through intravenous infusion, with an initial dosage of 35mg/kg for the first two administrations, followed by a maintenance dose of 50mg/kg every four weeks.122,123
Donanemab is an antibody designed to target the buildup of amyloid beta peptides (N3pG). It is given intravenously at doses of 700 to 1,400mg every four weeks. Clinical trials have not revealed significant concerns regarding fall risk, change in QTc interval, weight fluctuation, sedation, or vital signs. However, there have been observations of potential amyloid-related cerebral edema.124,125
Gantenerumab, a monoclonal antibody, specifically targets amyloid plaques by promoting their breakdown through microglial recruitment and phagocytosis. It is administered subcutaneously at different doses (120–1,200mg at minimum 1-week intervals). The FDA has recognized its potential by granting it a breakthrough designation.126
Simufilam (PTI-125) modulates the conformation and activity of filamin A, a structural protein that undergoes abnormal changes in Alzheimer’s disease, leading to the development and persistence of amyloid beta plaques. It acts as a filamin alpha-7 nicotinic acetylcholine receptor antagonist and is currently under investigation for the treatment of Alzheimer’s disease, with and without accompanying dementia. The recommended dosage is 100mg orally twice daily. While the drug has advanced to Phase III trials, concerns have been raised about the integrity of results. No serious adverse effects on health have been reported.127,128
Valiltramiprosate (ALZ-801) inhibits the aggregation of amyloid beta-42 through a mechanism involving 3-sulfopropanoic acid (3-SPA) metabolism. In early trials, oral administration of 265mg twice daily resulted in adverse effects, including nausea, dizziness, and vomiting.129
Other medications focus on modulating neurotransmitter systems, such as serotonin, GABA, and dopamine, which play crucial roles in cognitive function and psychiatric symptoms.129
ACP-204, an inverse agonist at 5-HT2A receptors, is being studied for Alzheimer’s disease-associated psychosis. Administered orally at 30 to 60mg daily, it does not cause QTc prolongation, unlike similar treatments.130,131
Pimavanserin (Nuplazid), a 5-HT2A and 5-HT2C inverse agonist, is being investigated for preventing relapse in dementia-related psychotic symptoms, residual psychotic symptoms in schizophrenia, and MDD. It is administered orally at 20mg daily and might prolong QTc intervals by 5 to 8ms. Currently, it is approved for Parkinson’s disease psychosis.132–134
Masupirdine, a 5-HT6 receptor selective antagonist, is being tested for Alzheimer’s disease. Commonly reported adverse effects include agitation, falls, and atrial fibrillation when administered orally at doses of 50 to 100mg daily.135
Suvorexant, an orexin-1 and orexin-2 receptor antagonist, was FDA-approved in 2014 for the treatment of insomnia and is being tested for the treatment of dementia. It is taken orally at night at a dose of 10mg, and somnolence is a commonly reported adverse effect.136,137
Latrepirdine (DMB-1, Dimebon), an H1 histamine receptor antagonist and NMDA receptor antagonist, is being studied for Alzheimer’s disease. Administered orally at doses of 10 to 60mg daily, it shows no significant risk of QTc prolongation, sedation, or falls.138
Levetiracetam (AGB101, Keppra), a synaptic vesicle glycoprotein 2A (SV2A) inhibitor, is being studied for mild cognitive impairment due to Alzheimer’s disease at low doses. Administered orally at 220mg daily, it showed no significant risk of QTc interval change or weight gain in Phase II trials, but falls were a common adverse effect.139,140
AR1001, a PDE5 inhibitor, is being investigated for mild cognitive impairment and early Alzheimer’s disease. The daily dosage is 30mg, taken orally. There are no significant risks related to QTc prolongation or weight gain, but fainting might increase the risk of falls.141
Dextromethorphan-bupropion (Auvelity, AXS-05) is being researched for treating agitation in Alzheimer’s disease and has been approved by the FDA for MDD. The medication combines dextromethorphan, which acts as an NMDA receptor antagonist and sigma-1 receptor agonist, with bupropion, a norepinephrine-dopamine reuptake inhibitor (NDRI) that competitively inhibits CYP2D6, resulting in a prolonged half-life of dextromethorphan to 22 hours. The recommended initial dosage is 45mg of dextromethorphan and 105mg of bupropion taken orally once daily, with a subsequent increase to twice daily after three days. Common side effects can include dizziness, headache, diarrhea, drowsiness, dry mouth, sexual dysfunction, and excessive sweating.142,143
Nabilone, a partial agonist at CB-1 and CB-2 cannabinoid receptors, is being studied for potential use in treating dementia. It is taken orally in doses ranging from 0.5 to 1.5mg daily and might cause sedation and drowsiness, increasing the risk of falls.144,145
Certain medications target mild cognitive impairment, early-stage Alzheimer’s disease, and related symptoms by affecting metabolic pathways and providing energy supplementation.
Tricaprilin, a medium-chain triglyceride metabolized to act as a ketogenic energy source, is being studied for Alzheimer’s disease. It is administered orally at 20g twice daily, and typical side effects include gastrointestinal discomfort and nausea.146
GV1001, a gonadotropin-releasing hormone receptor (GnRHR) activator, is being investigated for Alzheimer’s disease. When administered subcutaneously at doses ranging from 0.56 to 1.12mg weekly or biweekly, no notable adverse effects associated with QTc intervals, weight change, or fall risk were observed.147,148
Masitinib, a tyrosine kinase inhibitor, is currently under investigation for its potential to treat Alzheimer’s disease. In clinical studies, oral daily doses of 3 to 4.5mg/kg did not show significant risk in terms of changes to QTc interval and vital signs. However, notable adverse effects included neutropenia, hypoalbuminemia, and rash.149
Metformin extended release is being studied for its potential influence on cognitive function through the gut-brain axis. It regulates insulin levels, thus helping to prevent hyperinsulinemia, which has been associated with neuroinflammation and the accumulation of amyloid beta plaques. It is administered orally at doses ranging from 500 to 2,000mg daily. Some studies suggest a potential risk of cognitive decline in patients with Type 2 diabetes.150,151
Dapagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, decreases glucose reabsorption in blood and is being studied for cognitive impairment and dementia symptoms in patients with Type 2 diabetes. It is FDA-approved for Type 2 diabetes, heart failure, and chronic kidney disease and is administered at 10mg daily. Serious adverse effects include diabetic ketoacidosis and urinary tract infections.152
Nilotinib, a c-Abl tyrosine kinase receptor inhibitor, is being investigated for Alzheimer’s disease. When taken orally at 84 to 112mg daily, common side effects include mood swings, pain, and gastrointestinal discomfort. There are no reports of QTc prolongation, weight change, or fall risk.153,154
Xanomeline (Lumeron), a muscarinic receptor agonist selectively targeting M1 and M4 receptors, is being studied for Alzheimer’s disease and related cognitive deterioration. The daily oral dose ranges from 75 to 225mg, and it has been associated with weight gain and increased QTc interval at lower doses.155,156
Additional research is investigating the use of nanoparticles for the diagnosis and treatment of Alzheimer’s disease.157 Other potential treatments in earlier stages of clinical development remain focused on investigating disease-modifying therapies for various conditions (i.e., multiple sclerosis).158 See Table 12 for more details on each medication in the approval pipeline for neurocognitive disorders.
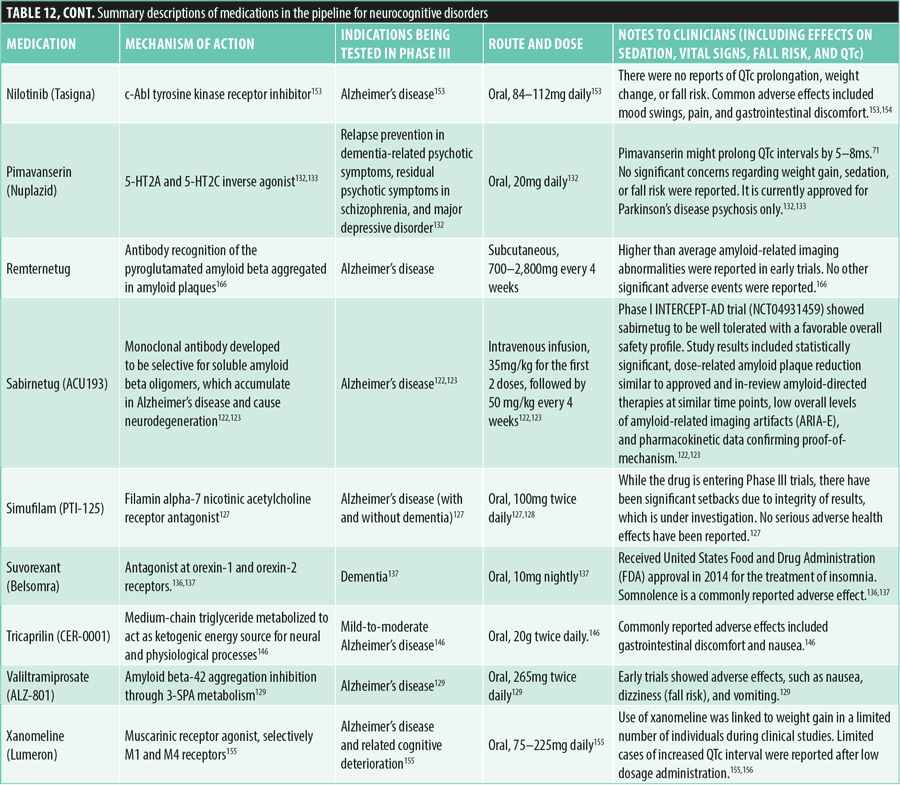
Neurodevelopmental disorders (attention deficit hyperactivity disorder [ADHD]). As of June 1, 2024, three pharmaceuticals had advanced to Phase III clinical trials for the treatment of neurodevelopmental disorders, specifically ADHD.
Centanafadine (EB-1020) is a SNDRI being studied for ADHD. It is administered orally at doses of 200 to 400mg daily and is well tolerated, with no significant risk of dependence, liver function issues, or sedation.159
NRCT-101SR exerts its effects on glutamatergic synapses to augment synaptic plasticity and neurotransmission. It is orally administered at doses ranging from 1,500 to 2,000mg per day, and it is well tolerated, with no significant adverse effects in clinical trials.160
Solriamfetol (Sunosi) is a dopamine and norepinephrine reuptake inhibitor and TAAR1 agonist approved for excessive daytime sleepiness due to OSA or narcolepsy. It is administered orally at doses of 150mg or 300mg daily. Common side effects include headache, nausea, and decreased appetite.161
Table 13 provides more detailed information on the medications in the pipeline for ADHD.
Discussion
This systematic review identified numerous psychiatric medications of potential interest that were in the late stages of development on the path to FDA approval as of June 1, 2024. A total of 90 pipeline drug trials were identified, including nine for schizophrenia, five for bipolar disorders, 25 for depressive disorders, 11 for anxiety disorders, five for PTSD, one for OCD, two for eating disorders, two for sleep-wake disorders, five for sexual dysfunctions, one for substance-related and addictive disorders, 22 for neurocognitive disorders, and three for neurodevelopmental disorders, specifically ADHD.
Significant activity is observed in the development of new medications for neurocognitive disorders. However, several agents were not reviewed in this manuscript, and some innovative mechanisms of action did not translate into significant clinical effects. For example, solanezumab, which is an intravenous monoclonal immunoglobulin G1 (IgG1) antibody targeting the mid-domain of the amyloid beta peptide for Alzheimer’s disease, did not meet its expected endpoint, leading to the discontinuation of its Phase III trials.162 Additionally, clinical trials on pimavanserin for schizophrenia were discontinued after they failed to demonstrate significant improvement in negative symptoms.163 Similarly, deudextromethorphan plus quinidine (AVP-786), which is an NMDA antagonist and sigma 1 receptor agonist and is FDA-approved for pseudobulbar affect, was studied in Phase III for agitation in dementia due to Alzheimer’s disease; it did not show significant difference from placebo and was thus terminated.164
Research on medications classified as psychedelic agents expanded to new indications, with several studies now in Phase III, and many others completing Phase II and approaching Phase III, such as ketamine for PTSD; psilocybin for alcohol use disorder, TRD, PTSD, GAD, OCD, fibromyalgia, and anorexia nervosa; and 5‐methoxy‐N, N‐dimethyltryptamine (5‐MeO‐DMT) for TRD, bipolar II disorder, postpartum depression, and alcohol use disorder.165 Most recently, lysergic acid diethylamide (LSD) was granted an FDA breakthrough designation for GAD; the Phase IIb trials of MM120 (lysergide d-tartrate) showed 65 percent response and 48 percent remission of GAD after 12 weeks of a single dose.166
Nutriband has partnered with Kindeva Drug Delivery to scale up the production of a new substance use medication that incorporates Nutriband’s AVERSA™ abuse-deterrent transdermal technology into Kindeva’s FDA-approved transdermal fentanyl patch system. The medication, AVERSA™ Fentanyl, aims to be the first abuse-deterrent patch of its kind and is currently in the process of obtaining FDA approval. Notably, the development has already completed a Phase I study, bypassing the need for Phase II and III trials.167 More progress is still being made with long-acting injectable antipsychotics, with subcutaneous monthly forms, such as olanzapine, being tested for efficacy.168
The field of psychiatry is in urgent need of innovation to effectively address the alarming rise of mental health disorders worldwide.169 The lack of effective psychiatric treatments and alternatives is reflected in the relatively high prevalence of treatment-resistant conditions. More research is needed to better understand the biopsychosocial mechanisms underlying mental disorders and the effects of medications.170,171 Historically, psychiatric disorders have been classified based on symptoms and diagnostic criteria. This poses a challenge, as patients diagnosed with the same disorder might present with a broad spectrum of phenotypes and clinical presentations. To address this challenge, biomarkers in psychiatry are a promising tool to help guide treatment for heterogeneous and complex mental disorders, as patients with the same diagnosis might respond differently to medications based on several mediating and moderating factors that continue to be identified at increasing rates. Several potential biomarkers have been identified and linked to certain neuropsychiatric conditions and neurodegenerative diseases.171 However, few have demonstrated to be useful in clinical practice to date.
Further exploration of the neurochemical pathways and associated biomarkers, in conjunction with comprehensive pharmaceutical reviews such as the present one, might consequently improve and optimize the clinical practice of pharmacotherapy. Many medications under development utilize well-established mechanisms of action. While innovative combinations, formulations, and applications could add significant value, there is also a pressing need for novel medications that achieve breakthroughs on our intolerably long list of refractory psychiatric conditions. However, without rigorous clinical trials, it is challenging to determine the efficacy of medications, their associated risks, and the most suitable treatments for each individual, which is crucial for achieving personalized medicine.
Strengths and limitations. This review’s strengths include a broad search strategy and the inclusion of multiple medication classes, which were systematically organized by psychiatric disorder. It identified medications for a wide range of psychiatric indications. This review also provides concise information for each medication, resulting in a practical resource. In addition, by highlighting mechanisms of action and dosing, this review reflects new trends and developments within the field.
Regarding limitations, this systematic review is limited by the available information and data made public by the agents behind these therapies. It is acknowledged that new medications are continuously being developed, and this review might not have captured all the upcoming developments.
Conclusion
We are entering a new era of psychiatry, which is reflected in the new and innovative treatments included in this review. To continue to advance the field and make a significant impact on the current worldwide burden of mental health conditions, it is critical to continue efforts in promoting innovation, development, and acceptance of new treatments. In this time of need, without efforts promoting research of new medications, there might be a significant delay in the development of new treatments and their impact on the general population.
References
- United States Food and Drug Administration. FDA drug approval process infographic (vertical). Current as of 26 Feb 2016. Accessed 1 May 2024. https://www.fda.gov/drugs/information-consumers-and-patients-drugs/fda-drug-approval-process-infographic-vertical
- Pahwa M, Sleem A, Elsayed OH, et al. New antipsychotic medications in the last decade. Curr Psychiatry Rep. 2021;23(12):87.
- Tsapakis EM, Diakaki K, Miliaras A, Fountoulakis KN. Novel compounds in the treatment of schizophrenia-a selective review. Brain Sci. 2023;13(8):1193.
- Greenwood LM, Leung S, Michie PT, et al. The effects of glycine on auditory mismatch negativity in schizophrenia. Schizophr Res. 2018;191:61–69.
- Kantrowitz JT, Epstein ML, Lee M, et al. Improvement in mismatch negativity generation during d-serine treatment in schizophrenia: correlation with symptoms. Schizophr Res. 2018;191:70–79.
- Rosenbrock H, Desch M, Wunderlich G. Development of the novel GlyT1 inhibitor, iclepertin (BI 425809), for the treatment of cognitive impairment associated with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2023;273(7):1557–1566.
- Kaul I, Sawchak S, Correll CU, et al. Efficacy and safety of the muscarinic receptor agonist KarXT (xanomeline-trospium) in schizophrenia (EMERGENT-2) in the USA: results from a randomised, double-blind, placebo-controlled, flexible-dose Phase 3 trial. Lancet. 2024;403(10422):160–170.
- Brannan SK, Sawchak S, Miller AC, et al. Muscarinic cholinergic receptor agonist and peripheral antagonist for schizophrenia. N Engl J Med. 2021;384(8):717–726.
- Højlund M, Correll CU. Ulotaront: a TAAR1/5-HT1A agonist in clinical development for the treatment of schizophrenia. Expert Opin Investig Drugs. 2022;31(12):1279–1290.
- Kuvarzin SR, Sukhanov I, Onokhin K, et al. Unlocking the therapeutic potential of ulotaront as a trace amine-associated receptor 1 agonist for neuropsychiatric disorders. Biomedicines. 2023;11(7):1977.
- Correll CU, Koblan KS, Hopkins SC, et al. Safety and effectiveness of ulotaront (SEP-363856) in schizophrenia: results of a 6-month, open-label extension study. NPJ Schizophr. 2021;7(1):63.
- Howes OD, Dawkins E, Lobo MC, et al. New drug treatments for schizophrenia: a review of approaches to target circuit dysfunction. Biol Psychiatry. 2024:S0006-3223(24)01349-0. Epub ahead of print.
- Achtyes ED, Hopkins SC, Dedic N, et al. Ulotaront: review of preliminary evidence for the efficacy and safety of a TAAR1 agonist in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2023;273(7):1543–1556.
- Corponi F, Fabbri C, Bitter I, et al. Novel antipsychotics specificity profile: a clinically oriented review of lurasidone, brexpiprazole, cariprazine and lumateperone. Eur Neuropsychopharmacol. 2019;29(9):971–985.
- ClinicalTrials.gov. Safety and tolerability trial of lumateperone in pediatric patients with schizophrenia or bipolar disorder. ClinicalTrials.gov Identifier: NCT06229210. Updated 30 Jan 2024. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT06229210
- Longo G, Cicolini A, Orsolini L, Volpe U. The novel antipsychotic lumateperone (ITI-007) in the treatment of schizophrenia: a systematic review. Brain Sci. 2023;13(12):1641.
- Kunz L. Phase 3 SOLARIS trial demonstrates potential of TEV-’749 in schizophrenia. Psychiatric Times. 9 May 2024. Accessed 6 Aug 2024. https://www.psychiatrictimes.com/view/phase-3-solaris-trial-demonstrates-potential-of-tev-749-in-schizophrenia
- Brand BA, de Boer JN, Marcelis MC, et al. The direct and long-term effects of raloxifene as adjunctive treatment for schizophrenia-spectrum disorders: a double-blind, randomized clinical trial. Schizophr Bull. 2023;49(6):1579–1590.
- Brand BA, de Boer JN, Oude Ophuis SBJ, et al. Raloxifene augmentation in men and women with a schizophrenia spectrum disorder: a study protocol. Contemp Clin Trials Commun. 2020;20:100681.
- Rabinowitz J, Staner C, Saoud J, et al. Long-term effects of roluperidone on negative symptoms of schizophrenia. Schizophr Res. 2023;255:9–13.
- Harvey PD, Saoud JB, Luthringer R, et al. Effects of Roluperidone (MIN-101) on two dimensions of the negative symptoms factor score: reduced emotional experience and reduced emotional expression. Schizophr Res. 2020;215:352–356.
- Moazen-Zadeh E, Bayanati S, Ziafat K, et al. Vortioxetine as adjunctive therapy to risperidone for treatment of patients with chronic schizophrenia: a randomised, double-blind, placebo-controlled clinical trial. J Psychopharmacol. 2020;34(5):506–513.
- Chen G, Højer AM, Areberg J, Nomikos G. Vortioxetine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet. 2018;57(6):673–686.
- ClinicalTrials.gov. Adjunctive vortioxetine in schizophrenia (AVIS). ClinicalTrials.gov Identifier: NCT02357797. Updated 8 Feb 2024. Accessed 2 Jun 2024. https://www.clinicaltrials.gov/study/NCT02357797
- ClinicalTrials.gov. Cognitive effects of adjuvant vortioxetine in early schizophrenia (CAVES). ClinicalTrials.gov Identifier: NCT04895488. Updated 26 Feb 2024. Accessed 2 Jun 2024. https://www.clinicaltrials.gov/study/NCT04895488
- Takeda Pharmaceuticals America, Inc. Trintellix (vortioxetine) tablets, for oral use: US prescribing information. Accessed 25 Mar 2024. https://content.takeda.com/?contenttype=PI&product=TRI&
language=ENG&country=GBL
&documentnumber=1 - Chen G, Lee R, Højer AM, et al. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig. 2013;33(10):727–736.
- Loebel A, Koblan KS, Tsai J, et al. A randomized, double-blind, placebo-controlled proof-of-concept trial to evaluate the efficacy and safety of non-racemic amisulpride (SEP-4199) for the treatment of bipolar I depression. J Affect Disord. 2022;296:
549–558. - Calabrese JR, Ketter TA, Youakim JM, et al. Adjunctive armodafinil for major depressive episodes associated with bipolar I disorder: a randomized, multicenter, double-blind, placebo-controlled, proof-of-concept study. J Clin Psychiatry. 2010;71(10):1363–1370.
- Ketter TA, Yang R, Frye MA. Adjunctive armodafinil for major depressive episodes associated with bipolar I disorder. J Affect Disord. 2015;181:87–91.
- Siwek M, Wojtasik-Bakalarz K, Krupa AJ, Chrobak AA. Brexpiprazole-pharmacologic properties and use in schizophrenia and mood disorders. Brain Sci. 2023;13(3):397.
- Nierenberg A, Lavin P, Javitt DC, et al. NRX-101 (D-cycloserine plus lurasidone) vs. lurasidone for the maintenance of initial stabilization after ketamine in patients with severe bipolar depression with acute suicidal ideation and behavior: a randomized prospective Phase 2 trial. Int J Bipolar Disord. 2023;11(1):28.
- ClinicalTrials.gov. Multicenter study of lumateperone for the treatment of bipolar depression in pediatric patients. ClinicalTrials.gov Identifier: NCT06372964. Updated 29 Jul 2024. Accessed 2 Jun 2024. https://clinicaltrials.gov/study/NCT06372964
- Sepanjnia K, Modabbernia A, Ashrafi M, et al. Pioglitazone adjunctive therapy for moderate-to-severe major depressive disorder: randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2012;37(9):2093–2100.
- Panikar V, Kale NJ, Hoskote SS, et al. Effect of low (7.5 mg/day), standard (15 mg/ day) and high (30 mg/day) dose pioglitazone therapy on glycemic control and weight gain in recently-diagnosed Type 2 diabetes patients. J Assoc Physicians India. 2015;63(11):36–39.
- Lett BT, Grant VL, Koh MT, Parsons JF. Chlordiazepoxide attenuates activity-induced anorexia and weight loss in rats. Exp Clin Psychopharmacol. 1998;6(4):360–366.
- Colle R, de Larminat D, Rotenberg S, et al. Pioglitazone could induce remission in major depression: a meta-analysis. Neuropsychiatr Dis Treat. 2016;13:9–16.
- Rajagopal L, Huang M, He W, et al. Repeated administration of rapastinel produces exceptionally prolonged rescue of memory deficits in phencyclidine-treated mice. Behav Brain Res. 2022;432:113964.
- Moskal JR, Burgdorf JS, Stanton PK, et al. The development of rapastinel (Formerly GLYX-13); a rapid acting and long lasting antidepressant. Curr Neuropharmacol. 2017;15(1):47–56.
- ClinicalTrials.gov. Study of rapastinel as monotherapy in patients with major depressive disorder (MDD). ClinicalTrials.gov Identifier: NCT03675776. Updated 28 Jul 2020. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT03675776
- ClinicalTrials.gov. Study of adjunctive or monotherapy rapastinel treatment in patients with major depressive disorder (MDD). ClinicalTrials.gov Identifier: NCT03668600. Updated 17 Jul 2020. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT03668600
- Savitz A, Wajs E, Zhang Y, et al. Efficacy and safety of seltorexant as adjunctive therapy in major depressive disorder: a Phase 2b, randomized, placebo-controlled, adaptive dose-finding study. Int J Neuropsychopharmacol. 2021;24(12):
965–976. - Recourt K, de Boer P, Zuiker R, et al. The selective orexin-2 antagonist seltorexant (JNJ-42847922/MIN-202) shows antidepressant and sleep-promoting effects in patients with major depressive disorder. Transl Psychiatry. 2019;9(1):216.
- Vasiliu O. Efficacy, tolerability, and safety of toludesvenlafaxine for the treatment of major depressive disorder-a narrative review. Pharmaceuticals (Basel). 2023;16(3):411.
- Li C, Jiang W, Gao Y, et al. Acute, subchronic oral toxicity, and genotoxicity evaluations of LPM570065, a new potent triple reuptake inhibitor. Regul Toxicol Pharmacol. 2018;98:129–139.
- Goodwin GM, Croal M, Feifel D, et al. Psilocybin for treatment resistant depression in patients taking a concomitant SSRI medication. Neuropsychopharmacology. 2023;48(10):1492–1499.
- ClinicalTrials.gov. Psilocybin-assisted therapy in treatment-resistant depression. ClinicalTrials.gov Identifier: NCT06303739. Updated 23 Jul 2024. Accessed 3 Jun 2024. https://classic.clinicaltrials.gov/ct2/show/record/NCT06303739
- Huang J, Pham M, Panenka WJ, et al. Chronic treatment with psilocybin decreases changes in body weight in a rodent model of obesity. Front Psychiatry. 2022;13:891512.
- Ekstrand J, Fattah C, Persson M, et al. Racemic ketamine as an alternative to electroconvulsive therapy for unipolar depression: a randomized, open-label, non-inferiority trial (KetECT). Int J Neuropsychopharmacol. 2022;25(5):339–349.
- Loo C, Glozier N, Barton D, et al. Efficacy and safety of a 4-week course of repeated subcutaneous ketamine injections for treatment-resistant depression (KADS study): randomised double-blind active-controlled trial. Br J Psychiatry. 2023;223(6):533–541.
- Tundo A, de Filippis R, De Crescenzo F. Pramipexole in the treatment of unipolar and bipolar depression. A systematic review and meta-analysis. Acta Psychiatr Scand. 2019;140(2):116–125.
- Goldberg JF, Burdick KE, Endick CJ. Preliminary randomized, double-blind, placebo-controlled trial of pramipexole added to mood stabilizers for treatment-resistant bipolar depression. Am J Psychiatry. 2004;161(3):564–566.
- Hori H, Kunugi H. The efficacy of pramipexole, a dopamine receptor agonist, as an adjunctive treatment in treatment-resistant depression: an open-label trial. ScientificWorldJournal. 2012;2012:372474.
- Martens MAG, Kaltenboeck A, Halahakoon DC, et al. An experimental medicine investigation of the effects of subacute pramipexole treatment on emotional information processing in healthy volunteers. Pharmaceuticals (Basel). 2021;14(8):800.
- Hampsey E, Jelen L, Young AH. Aticaprant: (a κ-opioid receptor antagonist) for major depressive disorder. Expert Opin Emerg Drugs. 2024;1–12. Epub ahead of print.
- ClinicalTrials.gov. A study of aticaprant as adjunctive therapy in adult participants with major depressive disorder (MDD) with moderate-to-severe anhedonia and inadequate response to current antidepressant therapy (VENTURA-1). ClinicalTrials.gov Identifier: NCT05455684. Updated 17 Jul 2024. Accessed 2 Jun 2024. https://clinicaltrials.gov/study/NCT05455684
- ClinicalTrials.gov. Study to assess the effects of oral NMRA-335140 in participants with major depressive disorder. ClinicalTrials.gov Identifier: NCT06058039. Updated 18 Jul 2024. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT06058039
- Namchuk AB, Lucki I, Browne CA. Buprenorphine as a treatment for major depression and opioid use disorder. Adv Drug Alcohol Res. 2022;2:10254.
- Peckham AM, De La Cruz A, Dufresne RL. Kappa opioid receptor antagonism: are opioids the answer for treatment resistant depression? Ment Health Clin. 2018;8(4):175–183.
- ClinicalTrials.gov. Effectiveness of cariprazine monotherapy for treatment of major depressive disorder. ClinicalTrials.gov Identifier: NCT05933538. Updated 7 Jul 2024. Accessed 3 Jun 2024. https://clinicaltrials.gov/ct2/show/NCT05933538
- Thorpy MJ, Shapiro C, Mayer G, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019;85(3):359–370. Erratum in: Ann Neurol. 2020;87(1):157.
- ClinicalTrials.gov. Progressing TAAR-1, dopamine, and norepinephrine in depression using solriamfetol (PARADIGM). ClinicalTrials.gov Identifier: NCT06360419. Updated 11 Apr 2024. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT06360419
- Bugarski-Kirola D, Arango C, Fava M, et al. Pimavanserin for negative symptoms of schizophrenia: results from the ADVANCE Phase 2 randomised, placebo-controlled trial in North America and Europe. Lancet Psychiatry. 2022;9(1):46–58.
- ClinicalTrials.gov. Adjunctive pimavanserin in subjects with major depressive disorder and inadequate response to antidepressant treatment. ClinicalTrials.gov Identifier: NCT03968159. Updated 17 Nov 2021. Accessed 3 Jun 2024. https://www.clinicaltrials.gov/study/NCT03968159
- ClinicalTrials.gov. Efficacy and safety of pimavanserin as adjunctive treatment for the negative symptoms of schizophrenia (ADVANCE-2). ClinicalTrials.gov Identifier: NCT04531982. Upodated 16 Aug 2023. Accessed 3 Jun 2024. https://www.clinicaltrials.gov/study/NCT04531982
- Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:
217–220. - Torres-Yaghi Y, Carwin A, Carolan J, et al. QTc interval prolongation with therapies used to treat patients with Parkinson’s disease psychosis: a narrative review. Neuropsychiatr Dis Treat. 2021;17:3791–3818.
- Tarzian M, Ndrio M, Chique B, et al. Illuminating hope for mental health: a drug review on lumateperone. Cureus. 2023;15(9):e46143.
- ClinicalTrials.gov. The efficacy and safety of mitizodone phosphate tablets in the treatment of patient with major depressive disorder. ClinicalTrials.gov Identifier: NCT04984512. Accessed 3 Jun 2024. https://www.clinicaltrials.gov/study/NCT04984512
- Fava M, Stahl SM, De Martin S, et al. Esmethadone-HCl (REL-1017): a promising rapid antidepressant. Eur Arch Psychiatry Clin Neurosci. 2023;273(7):1463–1476.
- Carr MM, Lou R, Macdonald-Gagnon G, et al. Weight change among patients engaged in medication treatment for opioid use disorder: a scoping review. Am J Drug Alcohol Abuse. 2023;49(5):551–565.
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):149–157.
- Mayo Clinic. Estradiol (vaginal route). Updated 1 Jul 2024. Accessed 3 Jun 2024. https://www.mayoclinic.org/drugs-supplements/estradiol-vaginal-route/side-effects/drg-20075648?p=1
- Costi S, Morris LS, Kirkwood KA, et al. Impact of the KCNQ2/3 channel opener ezogabine on reward circuit activity and clinical symptoms in depression: results from a randomized controlled trial. Am J Psychiatry. 2021;178(5):437–446.
- Clayton AH, Lasser R, Nandy I, et al. Zuranolone in major depressive disorder: results from MOUNTAIN-a Phase 3, multicenter, double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2023;84(2):22m14445.
- Halaris A. Inflammation and depression but where does the inflammation come from? Curr Opin Psychiatry. 2019;32(5):422–428.
- Gędek A, Szular Z, Antosik AZ, et al. Celecoxib for mood disorders: a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2023;12(10):3497.
- Wu KJ, Wang YS, Hung TW, et al. Effect of celecoxib on improving depression: a systematic review and meta-analysis. World J Clin Cases. 2022;10(22):7872–7882.
- ClinicalTrials.gov. Treating immuno-metabolic depression with anti-inflammatory drugs (INFLAMED). ClinicalTrials.gov Identifier: NCT05415397. Updated 25 Sep 2023. Accessed 2 Jun 2024. https://www.clinicaltrials.gov/study/NCT05415397
- ClinicalTrials.gov. Inflammation-based stratification for immune-targeted augmentation in major depressive disorder (INSTA-MD). Updated 9 Dec 2022. ClinicalTrials.gov Identifier: NCT05644301. Accessed 2 Jun 2024. https://www.clinicaltrials.gov/study/NCT05644301
- ClinicalTrials.gov. The effect of celecoxib on neuroinflammation in MDD. ClinicalTrials.gov Identifier: NCT04814355. Updated 22 Nov 2023. Accessed 2 Jun 2024. https://www.clinicaltrials.gov/study/NCT04814355
- Avari JN, Kanellopoulos D, Solomonov N, et al. Minocycline augmentation in older adults with persistent depression: an open label proof of concept study. Int Psychogeriatr. 2020;32(7):881–884.
- Soczynska JK, Kennedy SH, Alsuwaidan M, et al. A pilot, open-label, 8-week study evaluating the efficacy, safety and tolerability of adjunctive minocycline for the treatment of bipolar I/II depression. Bipolar Disord. 2017;19(3):198–213.
- Stein DJ, Ahokas A, Márquez MS, et al. Agomelatine in generalized anxiety disorder: an active comparator and placebo-controlled study. J Clin Psychiatry. 2014;75(4):362–368.
- Kozian R, Syrbe G. QTc-Zeit-Verlängerung unter Therapie mit Agomelatin [QTc prolongation during treatment with agomelatine]. Psychiatr Prax. 2010;37(8):405–407. German.
- ClinicalTrials.gov. Safety and Efficacy evaluation of pregabalin (Lyrica) with patients with generalized anxiety disorder. ClinicalTrials.gov Identifier: NCT00624780. Updated 28 Jan 2021. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT00624780
- Hamed SA. Sexual dysfunctions induced by pregabalin. Clin Neuropharmacol. 2018;41(4):116–122.
- Maneeton N, Maneeton B, Woottiluk P, et al. Quetiapine monotherapy in acute treatment of generalized anxiety disorder: a systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2016;10:259–276.
- Katzman MA, Brawman-Mintzer O, Reyes EB, et al. Extended release quetiapine fumarate (quetiapine XR) monotherapy as maintenance treatment for generalized anxiety disorder: a long-term, randomized, placebo-controlled trial. Int Clin Psychopharmacol. 2011;26(1):11–24.
- Liebowitz MR, Salman E, Nicolini H, et al. Effect of an acute intranasal aerosol dose of PH94B on social and performance anxiety in women with social anxiety disorder. Am J Psychiatry. 2014;171(6):675–682.
- ClinicalTrials.gov. Fasedienol nasal spray for the acute treatment of anxiety in adults with social anxiety disorder (PALISADE-3) (PALISADE-3). ClinicalTrials.gov Identifier: NCT06358651. Updated 24 Jul 2024. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT06358651
- Saletu-Zyhlarz GM, Anderer P, Wolzt M, et al. Double-blind, placebo-controlled, multiple-ascending-dose study on the pharmacodynamics of ABIO-08/01, a new CNS drug with potential anxiolytic activity. 1. EEG mapping, psychometric and tolerability findings. Neuropsychobiology. 2009;59(2):100–109.
- Yang F, Wang B, Liu Z, et al. Prediction of a therapeutic dose for buagafuran, a potent anxiolytic agent by physiologically based pharmacokinetic/pharmacodynamic modeling starting from pharmacokinetics in rats and human. Front Pharmacol. 2017;8:683.
- Stemmelin J, Cohen C, Terranova JP, et al. Stimulation of the beta3-adrenoceptor as a novel treatment strategy for anxiety and depressive disorders. Neuropsychopharmacology. 2008;33(3):
574–587. - ClinicalTrials.gov. A clinical study that will measure how well SEP-363856 works and how safe it is in adults with generalized anxiety disorder. ClinicalTrials.gov Identifier: NCT05729373. Updated 26 Jun 2024. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT05729373
- ClinicalTrials.gov. Relapse-prevention study with Lu AA21004 (vortioxetine) in patients with generalized anxiety disorder. ClinicalTrials.gov Identifier: NCT00788034. Updated 23 Jun 2015. Accessed 3 Jun 2024. https://classic.clinicaltrials.gov/ct2/show/NCT00788034
- Parmenter ME, Lederman S, Weathers FW, et al. A Phase 3, randomized, placebo-controlled, trial to evaluate the efficacy and safety of bedtime sublingual cyclobenzaprine (TNX-102 SL) in military-related posttraumatic stress disorder. Psychiatry Res. 2024;334:115764.
- Togno J, Eaton S. Is there a role for prazosin in the treatment of post-traumatic stress disorder? Aust Fam Physician. 2015;44(9):647–649.
- Paiva HS, Filho IJZ, Cais CFDS. Using prazosin to treat posttraumatic stress disorder and associations: a systematic review. Psychiatry Investig. 2021;18(5):365–372.
- Nugent NR, Christopher NC, Crow JP, et al. The efficacy of early propranolol administration at reducing PTSD symptoms in pediatric injury patients: a pilot study. J Trauma Stress. 2010;23(2):282–287.
- Strawn JR, Keeshin BR, DelBello MP, et al. Psychopharmacologic treatment of posttraumatic stress disorder in children and adolescents: a review. J Clin Psychiatry. 2010;71(7):932–941.
- Shiner B, Huybrechts K, Gui J, et al. Comparative effectiveness of direct-acting antivirals for posttraumatic stress disorder in Veterans Affairs patients with hepatitis C virus infection. Am J Epidemiol. 2022;191(9):
1614–1625. - AbbVie. Common side effects of Mavyret. Accessed 6 Aug 2024. https://www.mavyret.com/side-effects
- Tedesco S, Gajaram G, Chida S, et al. The efficacy of MDMA (3,4-methylenedioxymethamphetamine) for post-traumatic stress disorder in humans: a systematic review and meta-analysis. Cureus. 2021;13(5):e15070.
- Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165(7):917–928.
- Pittenger C. Glutamatergic agents for OCD and related disorders. Curr Treat Options Psychiatry. 2015;2(3):271–283.
- ClinicalTrials.gov. Efficacy and safety study of adjunctive troriluzole in obsessive compulsive disorder. ClinicalTrials.gov Identifier: NCT04641143. Updated 19 Apr 2024. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT04641143
- Grilo CM, Lydecker JA, Jastreboff AM, et al. Naltrexone/bupropion for binge-eating disorder: a randomized, double-blind, placebo-controlled trial. Obesity (Silver Spring). 2023;31(11):2762–2773.
- Krystal AD, Benca RM, Rosenberg R, et al. Solriamfetol treatment of excessive daytime sleepiness in participants with narcolepsy or obstructive sleep apnea with a history of depression. J Psychiatr Res. 2022;155:
202–210. - ClinicalTrials.gov. Elucidating TAAR-1, dopamine, and norepinephrine in binge eating disorder using solriamfetol (ENGAGE). ClinicalTrials.gov Identifier: NCT06413433. Updated 14 May 2024. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT06413433
- Roth T, Thorpy MJ, Kushida CA, et al. Once-nightly sodium oxybate (FT218) improved symptoms of disrupted nighttime sleep in people with narcolepsy: a plain language summary. J Comp Eff Res. 2023;12(12):e230133.
- Avadel CNS Pharmaceuticals, LLC. MED-US-LUM-2100001 Risk Evaluation and Mitigation Strategy (REMS) Document LUMRYZ TM (sodium oxybate extended-release) REMS Program. 2023. Accessed 3 Jun 2024. https://www.accessdata.fda.gov/drugsatfda_docs/REMS/214755Orig1s000REMS.pdf
- Parkes JD, Schachter M. Mazindol in the treatment of narcolepsy. Acta Neurol Scand. 1979;60(4):250–254.
- Roehrborn CG, Manyak MJ, Palacios-Moreno JM, et al. A prospective randomised placebo-controlled study of the impact of dutasteride/tamsulosin combination therapy on sexual function domains in sexually active men with lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH). BJU Int. 2018;121(4):647–658.
- ClinicalTrials.gov. Prospective sexual function study for BPH subjects. ClinicalTrials.gov Identifier: NCT01777269. Updated 20 Aug 2018. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT01777269
- Giuliano F, Joussain C, Denys P. Long-term effectiveness and safety of intracavernosal botulinum toxin A as an add-on therapy to phosphodiesterase type 5 inhibitors or prostaglandin E1 injections for erectile dysfunction. J Sex Med. 2022;19(1):83–89.
- Abdelrahman IFS, Raheem AA, Elkhiat Y, et al. Safety and efficacy of botulinum neurotoxin in the treatment of erectile dysfunction refractory to phosphodiesterase inhibitors: results of a randomized controlled trial. Andrology. 2022;10(2):254–261.
- Hosny KM, El-Say KM, Ahmed OA. Optimized sildenafil citrate fast orodissolvable film: a promising formula for overcoming the barriers hindering erectile dysfunction treatment. Drug Deliv. 2016;23(1):355–361.
- Loprete L, Leuratti C, Frangione V, Radicioni M. Pharmacokinetics of a novel sildenafil orodispersible film administered by the supralingual and the sublingual route to healthy men. Clin Drug Investig. 2018;38(8):765–772.
- de Beaurepaire R, Sinclair JMA, Heydtmann M, et al. The use of baclofen as a treatment for alcohol use disorder: a clinical practice perspective. Front Psychiatry. 2019;9:708.
- Zhang Y, Chen H, Li R, et al. Amyloid β-based therapy for Alzheimer’s disease: challenges, successes and future. Signal Transduct Target Ther. 2023;8(1):248.
- Acumen Pharmaceuticals. Acumen Pharmaceuticals announces first patient Dosed in ALTITUDE-AD, a Phase 2 clinical trial of sabirnetug (ACU193) in early Alzheimer’s disease. 8 May 2024. Accessed 3 Jun 2024. https://investors.acumenpharm.com/news-releases/news-release-details/acumen-pharmaceuticals-announces-first-patient-dosed-altitude-ad
- ClinicalTrials.gov. A study to evaluate efficacy and safety of intravenous ACU193 in oarticipants with early Alzheimer’s disease (ALTITUDE-AD) (ALTITUDE-AD). ClinicalTrials.gov Identifier: NCT06335173. Updated 6 Aug 2024. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT06335173
- Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691–1704.
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512–527.
- Klein G, Delmar P, Voyle N, et al. Gantenerumab reduces amyloid-β plaques in patients with prodromal to moderate Alzheimer’s disease: a PET substudy interim analysis. Alzheimers Res Ther. 2019;11(1):101.
- Wang HY, Cecon E, Dam J, et al. Simufilam reverses aberrant receptor interactions of filamin A in Alzheimer’s disease. Int J Mol Sci. 2023;24(18):13927.
- Wang HY, Pei Z, Lee KC, et al. PTI-125 reduces biomarkers of Alzheimer’s disease in patients. J Prev Alzheimers Dis. 2020;7(4):256–264.
- Hey JA, Kocis P, Hort J, et al. Discovery and identification of an endogenous metabolite of tramiprosate and its prodrug ALZ-801 that inhibits beta amyloid oligomer formation in the human brain. CNS Drugs. 2018;32(9):849–861. Erratum in: CNS Drugs. 2018;32(12):1185.
- ClinicalTrials.gov. ACP-204 in adults with Alzheimer’s disease psychosis. ClinicalTrials.gov Identifier: NCT06159673. Updated 1 Aug 2024. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT06159673
- ClinicalTrials.gov. ACP-204 in adults with Alzheimer’s disease psychosis open label extension study. ClinicalTrials.gov Identifier: NCT06194799. Updated 2 Aug 2024. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT06194799
- Cummings J, Ballard C, Tariot P, et al. Pimavanserin: Potential treatment for dementia-related psychosis. J Prev Alzheimers Dis. 2018;5(4):253–258.
- Tariot PN, Cummings JL, Soto-Martin ME, et al. Trial of pimavanserin in dementia-related psychosis. N Engl J Med. 2021;385(4):
309–319. - ClinicalTrials.gov. Relapse prevention study of pimavanserin in dementia-related psychosis. ClinicalTrials.gov Identifier: NCT03325556. Updated 21 Jun 2021. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT03325556
- Nirogi R, Ieni J, Goyal VK, et al. Effect of masupirdine (SUVN-502) on cognition in patients with moderate Alzheimer’s disease: a randomized, double-blind, Phase 2, proof-of-concept study. Alzheimers Dement (N Y). 2022;8(1):e12307.
- Herring WJ, Ceesay P, Snyder E, et al. Polysomnographic assessment of suvorexant in patients with probable Alzheimer’s disease dementia and insomnia: a randomized trial. Alzheimers Dement. 2020;16(3):541–551.
- Owen RT. Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barc). 2016;52(1):29–40.
- Bezprozvanny I. The rise and fall of Dimebon. Drug News Perspect. 2010;23(8):518–523.
- ClinicalTrials.gov. AGB101 for mild cognitive impairment. ClinicalTrials.gov Identifier: NCT05986721. Updated 20 Jun 2024. Accessed 20 Jun 2024. https://clinicaltrials.gov/study/NCT05986721
- Mohs R, Bakker A, Rosenzweig-Lipson S, et al. The HOPE4MCI study: a randomized double-blind assessment of AGB101 for the treatment of MCI due to AD. Alzheimers Dement (N Y). 2024;10(1):e12446.
- Kang BW, Kim F, Choi YP, et al. AR1001 ameliorates Alzheimer’s disease pathology and symptoms by multi-mechanisms. Alzheimers Dement. 2020;16:e047266.
- Putka S. Alzheimer’s agitation relapse delayed with dextromethorphan-bupropion. MedPage Today. Updated 18 Apr 2024. Accessed 7 Aug 2024. https://www.medpagetoday.com/meetingcoverage/aan/109714
- McCarthy B, Bunn H, Santalucia M, et al. Dextromethorphan-bupropion (Auvelity) for the treatment of major depressive disorder. Clin Psychopharmacol Neurosci. 2023;21(4):609–616.
- Bajtel Á, Kiss T, Tóth B, et al. The safety of dronabinol and nabilone: a systematic review and meta-analysis of clinical trials. Pharmaceuticals (Basel). 2022;15(1):100.
- Herrmann N, Ruthirakuhan M, Gallagher D, et al. Randomized placebo-controlled trial of nabilone for agitation in Alzheimer’s disease. Am J Geriatr Psychiatry. 2019;27(11):
1161–1173. - Li Z, Ramirez G, Tang R, et al. Modeling digestion, absorption, and ketogenesis after administration of tricaprilin formulations to humans. Eur J Pharm Biopharm. 2023;182:
41–52. - Koh SH, Kwon HS, Choi SH, et al. Efficacy and safety of GV1001 in patients with moderate-to-severe Alzheimer’s disease already receiving donepezil: a Phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. Alzheimers Res Ther. 2021;13(1):66.
- Park H, Kwon HS, Lee KY, et al. GV1001 modulates neuroinflammation and improves memory and behavior through the activation of gonadotropin-releasing hormone receptors in a triple transgenic Alzheimer’s disease mouse model. Brain Behav Immun. 2024;115:295–307.
- Dubois B, López-Arrieta J, Lipschitz S, et al. Masitinib for mild-to-moderate Alzheimer’s disease: results from a randomized, placebo-controlled, Phase 3, clinical trial. Alzheimers Res Ther. 2023;15(1):39. Erratum in: Alzheimers Res Ther. 2023;15(1):85.
- Campbell JM, Stephenson MD, de Courten B, et al. Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J Alzheimers Dis. 2018;65(4):1225–1236.
- Rosell-Díaz M, Fernández-Real JM. Metformin, cognitive function, and changes in the gut microbiome. Endocr Rev. 2024;45(2):210–226.
- Samman WA, Selim SM, El Fayoumi HM, et al. Dapagliflozin ameliorates cognitive impairment in aluminum-chloride-induced Alzheimer’s disease via modulation of AMPK/mTOR, oxidative stress and glucose metabolism. Pharmaceuticals (Basel). 2023;16(5):753.
- Turner RS, Hebron ML, Lawler A, et al. Nilotinib effects on safety, tolerability, and biomarkers in Alzheimer’s disease. Ann Neurol. 2020;88(1):183–194.
- Nobili A, D’Amelio M, Viscomi MT. Nilotinib: from animal-based studies to clinical investigation in Alzheimer’s disease patients. Neural Regen Res. 2023;18(4):803–804.
- Mirza NR, Peters D, Sparks RG. Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists. CNS Drug Rev. 2003;9(2):159–186.
- Brannan SK, Sawchak S, Miller AC, et al. Muscarinic cholinergic receptor agonist and peripheral antagonist for schizophrenia. N Engl J Med. 2021;384(8):717–726.
- Puranik N, Yadav D, Song M. Advancements in the application of nanomedicine in Alzheimer’s disease: a therapeutic perspective. Int J Mol Sci. 2023;24(18):14044.
- Morató X, Pytel V, Jofresa S, et al. Symptomatic and disease-modifying therapy pipeline for Alzheimer’s disease: towards a personalized polypharmacology patient-centered approach. Int J Mol Sci. 2022;23(16):9305.
- Adler LA, Adams J, Madera-McDonough J, et al. Efficacy, safety, and tolerability of centanafadine sustained-release tablets in adults with attention-deficit/hyperactivity disorder: results of 2 Phase 3, randomized, double-blind, multicenter, placebo-controlled trials. J Clin Psychopharmacol. 2022;42(5):429–439.
- ClinicalTrials.gov. Study to evaluate NRCT-101SR in pediatric subjects with ADHD (ADHD). ClinicalTrials.gov Identifier: NCT06215144. Updated 20 Mar 2024. Accessed 3 Jun 2024. https://clinicaltrials.gov/study/NCT06215144
- Surman CBH, Walsh DM, Horick N, et al. Solriamfetol for attention-deficit/hyperactivity disorder in adults: a double-blind placebo-controlled pilot study. J Clin Psychiatry. 2023;84(6):23m14934.
- Sperling RA, Donohue MC, Raman R, et al. Trial of solanezumab in preclinical Alzheimer’s disease. N Engl J Med. 2023;389(12):
1096–1107. - Medscape. Acadia to stop trials of antipsychotic drug after it fails schizophrenia study. 11 Mar 2024. Accessed 3 Jun 2024. https://www.medscape.com/s/viewarticle/acadia-stop-trials-antipsychotic-drug-after-it-fails-2024a10004m5
- Lobo A. Otsuka stops developing AVP-786 therapy for Alzheimer’s agitation. Alzheimer’s News Today. 23 May 2024. Accessed 3 Jun 2024. https://alzheimersnewstoday.com/news/otsuka-stops-development-avp-786-agitation-alzheimers/
- Psychedelic Alpha. Psychedelic drug development tracker. Accessed 3 Jun 2024. https://psychedelicalpha.com/data/psychedelic-drug-development-tracker
- MindMed. MindMed receives FDA breakthrough therapy designation and announces positive 12-week durability data from Phase 2B study of MM120 for generalized anxiety disorder. 7 Mar 2024. Accessed 3 Jun 2024. https://ir.mindmed.co/news-events/press-releases/detail/137/mindmed-receives-fda-breakthrough-therapy-designation-and-announces-positive-12-week-durability-data-from-phase-2b-study-of-mm120-for-generalized-anxiety-disorder
- BioSpace. Nutriband provides clinical and regulatory path overview for lead product – AVERSA® Fentanyl transdermal patch. 27 Mar 2024. Accessed 3 Jun 2024. https://www.biospace.com/article/releases/nutriband-provides-clinical-and-regulatory-path-overview-for-lead-product-aversa-r-fentanyl-transdermal-patch/
- Kunz L. Phase 3 SOLARIS trial demonstrates potential of TEV-’749 in schizophrenia. Psychiatric Times. 9 May 2024. Accessed 7 Aug 2024. https://www.psychiatrictimes.com/view/phase-3-solaris-trial-demonstrates-potential-of-tev-749-in-schizophrenia
- Sarris J. Disruptive innovation in psychiatry. Ann N Y Acad Sci. 2022;1512(1):5–9.
- Markowitz JC. Supportive evidence: brief supportive psychotherapy as active control and clinical intervention. Am J Psychother. 2022;75(3):122–128.
- García-Gutiérrez MS, Navarrete F, Sala F, et al. Biomarkers in psychiatry: concept, definition, types and relevance to the clinical reality. Front Psychiatry. 2020;11:432.



