 by Rocco S. Calabrò, MD, PhD; Placido Bramanti, MD; Annalisa Baglieri, PsyD, PhD; Francesco Corallo, PsyD; Rosaria De Luca, MSc, PhD; Simona De Salvo, MSc; Silvia Marino, MD, PhD
by Rocco S. Calabrò, MD, PhD; Placido Bramanti, MD; Annalisa Baglieri, PsyD, PhD; Francesco Corallo, PsyD; Rosaria De Luca, MSc, PhD; Simona De Salvo, MSc; Silvia Marino, MD, PhD
All from IRCCS Centro Neurolesi Bonino-Pulejo, Messina, Italy
Innov Clin Neurosci. 2015;12(1–2):24–28.
Funding: No funding was provided for the preparation of this article.
Financial disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Background: Functional studies have been previous reported in stroke patients, but no studies of functional magnetic resonance imaging have been performed in Moyamoya disease. Objective: To assess the cortical and cerebellar reorganization in a moyamoya patient. Methods: We reported a case of a patient suffering from moyamoya disease, undergoing a neuropsychological assessment, a neurocognitive rehabilitative treatment, an electroencephalogram evaluation, and a functional magnetic resonance imaging examination. Results: The subject showed a cognitive impairment, a slow electroencephalogram activity, and the ipsi- and controlateral motor cortex and cerebellar functional magnetic resonance imaging activation. Conclusions: This is the first functional magnetic resonance imaging case study reported in moyamoya disease. We showed a cortical reorganization, which could play an important role in clinical evaluation and motor recovery. The cerebellar activation, showed after cognitive and motor rehabilitation, could support the idea that the cerebellum contains several cognitive-related subregions involved in different functional networks in moyamoya disease.
Key words: Cerebellar reorganization, cortical reorganization, functional magnetic resonance imaging, fMRI, moyamoya disease, rehabilitation
Introduction
Cerebrovascular moyamoya disease is characterized by progressive stenotic changes in the terminal portion of the bilateral internal carotid arteries and the formation of an abnormal vascular network at the base of the brain. The latter is thought to be a secondary phenomenon that compensates for the cerebral ischemia due to the primary internal carotid artery stenosis. The abnormally developed vascular network is defined as “moyamoya vessels.”[1,2] Many new aspects of the epidemiology have been uncovered so far, and innovations in surgical treatment have been developed, including direct bypass surgery and other combined revascularization treatments.[3]
Several hypotheses concerning the pathogenesis of moyamoya disease have been proposed, such as autoimmune and human leukocyte antigen (HLA) abnormalities—susceptible genetic loci for familial moyamoya disease. Moreover, interesting works have shown the potential role of endothelial progenitor cells on the pathogenesis of moyamoya disease.[4]
In children, the most common presentation is mainly related to the cerebral ischemia, with recurrent episodes of hemiparesis. Nevertheless, convulsions, headaches, and paraesthesias may also occur. Adults, indeed, present most commonly with subarachnoid, intracerebral, or intraventricular hemorrhage.[1,5]
Structural imaging of the brain with computed tomography (CT), magnetic resonance imaging (MRI), MR angiography, and conventional angiography is capable in identifying areas of parenchymal injury and large vessel occlusion. These imaging modalities, also by using a high-field scanner and electroencephalographic (EEG) examinations, provide information in understanding the pathophysiology of vascular diseases.[6–8]
Functional magnetic resonance imaging (fMRI) is a noninvasive MRI technique, which was also used to map regions of brain activation during motor tasks in stroke patients: it can define abnormal pattern of activation in disease.
In this case study, by using a high-field MR scanner, we investigated whether such adaptation is demonstrable using fMRI during repeated hand movements in a simple and well-learned motor task. To date, no fMRI case studies in moyamoya patients have been reported.
Case Report
A 40-year-old man presented to our neurorehabilitation unit after suffering a recurrent ischemic stroke. He had a history of tabagism and alcoholism with episodes of pancreatitis; he was, moreover, affected by diabetes mellitus treated with insulin, and borderline hypertension.
In April 2010, he was admitted to the neurologic unit for sudden onset of right hemiparesis due to a cerebral infarction. After a month, he presented with a stroke recurrence with a more severe right hemiparesis and moderate primarily motor aphasia. MRI evidenced an ischemic lesion in the left fronto-temporal area. Somatosensory, motor- and visual-evoked potentials, as well as liquor analysis were within the normal range. Anti-nuclear antibodies were moderately positive (1:80). For this reason, cerebral vasculitis was suspected and the patient was prescribed antiplatelets and cortisone therapy, which was withdrawn after two months for side effects (i.e. hyperglycemia). The patient was referred to our institute for an intensive neurorehabilitative treatment three months after the first stroke. During the neurological examination, he showed moderate hemiparesis with a mild distal spasticity of the upper limbs and a moderate aphasia.
Cognitive disability was investigated through a proper psychometric battery, including Mini Mental State Examination (MMSE; 22.43), Levels of Cognitive Functioning (LCF, 7, Category Verbal Fluency (CVF, 8), Letter Verbal Fluency (LVF, 23), Attentive Matrices (AM, 24,25), Trial making test (TMT-A, 77), TMT-B (229), TMT B-A (306), Reversal Motor Learning (RML, 20), Rey Auditory Verbal Learning immediate (RAVLI, 25.2), and delayed-RAVLR (6.30) recall, Digit Test (DT, 2.75), and constructive apraxia (AC, 13). All tests were administered at baseline and two months after a specific cognitive pc-training tool execution, in addition to the conventional rehabilitation. The neuropsychological evaluation showed attention and memory impairments, with a moderate alteration in verbal fluency and executive process. Interestingly, we have observed a post-treatment cognitive global improvement in LCF (8) and in MMSE (25), with a significant increase in memory and attention abilities (DT, 4; RAVLI, 33.5; RAVLR, 7.7; AM, 33), language functions (CVF, 15; LVF, 30), learning process (RML, 24), constructional apraxia (CA, 19.75) and executive functioning (TMT-B, 160; TMT B-A, 90).
During hospitalization he presented several episodes characterized by a sudden worsening of motor impairment with global aphasia, lasting from 30 to 180 minutes: consciousness was not altered and such symptomatology recovered slowly. EEG evaluation showed a predominantly alpha activity background (9–10Hz) with a theta activity (5–7Hz) on the left fronto-temporal areas (data not shown). The patient underwent a conventional brain MRI that showed multiple and circumscribed areas of hyperintensities in the bilateral fronto-parietal regions within of bilateral periventricular white matter and in the context of left semi-oval center.
Moreover, the patient underwent an MR angiography, which showed stenosis of the bilateral internal carotid arteries associated with basal moyamoya vessels; middle cerebral artery and anterior cerebral artery were also discontinuous (Suzuki’s conventional angiography stage III on both sides) (data not shown). Thus, the patient was referred to a neurosurgery center where he underwent surgical revascularization using an indirect bypass to improve cerebral hemodynamics and to reduce the risk of subsequent stroke.
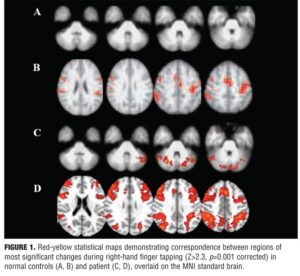
The patient then came back to our center to continue his intensive neurorehabilitative program. When the motor and cognitive functions improved, the patient and five sex- and age-matched normal controls (NC) consented to undergo an fMRI examination on a system operating at 3.0 T (Philips, Achieva, The Netherlands). Blood oxygen-dependent contrast echo planar imaging scans were performed. All subjects were instructed to perform a simple motor task using a “block” design in which 30 seconds of finger-tapping with right hand was alternated with a 30-second rest period. The rate of tapping was 1Hz for each individual. We used a finger tapping task because it is well known for assessing motor impairment after stroke: in fact, this method is thought to reflect corticospinal tract damage,[9] the main cause of permanent disability after stroke. The data were analyzed and processed using an advanced image analysis tools from the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (available at http://www.fmrib.ox.ac.uk/fsl). Cluster detection was undertaken on voxels at a threshold of Z>2.3. A corrected probability threshold of p=0.001 was then applied to determine significant clusters, and the position of the local maxima in each independent activation cluster was defined.
We noted a functional activation pattern localized in the controlateral and ipsilateral motor areas (premotor cortex, primary motor cortex, and supplementary motor area) in the patient. The NC showed the activation of controlateral motor areas. In addition, the patient showed cerebellar area activation when compared to the NC (corrected p value <0.001 for all regions) (Figure 1).
To statistically compare activation, a standard analysis framework was performed in which the percentage voxels activated, and the average Z scores of activated voxels in each ROI were used as measures of activation. The distributions were significantly different (Kolmogorov–Smirnov test, D=0.47, p<0.001) with means of -0.226±1.01.
Discussion
Moyamoya phenomenon is likely a secondary response to many different causes of arterial stenosis and occlusion.[1,2] Indeed, previous works have recognized that atherosclerosis may be one of a number of causative factors for this manifestation. Clinical findings of our patient, were consistent to those previously reported in the literature.[1–3] EEG changes reported in Moyamoya include posterior slowing and centrotemporal slow activity. Our patient’s EEG showed a slow periodic non-epileptiform activity predominantly in the left fronto-temporal areas, and presented several ictal episodes, which we were not completely able to identify as either epileptic or syncopal attacks. However, based on our patient’s clinical and EEG data, this symptomatology seems to be related to cerebral hypoperfusion.
Cognitive impairment, with regard to dysexecutive syndrome, is a frequent complication of Moyamoya in adults, and it may be related to hypoperfusion in frontal areas. Several authors have demonstrated improvement of cognitive functions after revascularization to frontal lobes with and without infarctions.[6,7] Our patient underwent a specific motor and cognitive rehabilitative program, after the surgical treatment, with a global motor and cognitive good recovery (p<0.05).
Application of novel imaging techniques and high-resolution magnets, like the scanner we used, have significantly improved MR resolution, contrast, and signal-to-noise ratio such that visualization of lesional components is now feasible.[10–13] The morbidity of Moyamoya disease is directly related to the cerebral blood flow, which appears to decrease especially in the frontal region with relatively normal flow in the temporal and occipital areas. Previous positron emission tomographic reports showed an increase in total blood volume, especially in the striatum.[14] Other studies have demonstrated that reorganization of the cortex after stroke contributed to the motor recovery.[15–17]
It is well know that the physiotherapic treatment could increase the cortical and subcortical areas involved in the movement, especially in stroke patients. In fact, rehabilitation practice-induced neuroplasticity and behavioral gains have been repeatedly demonstrated for the upper and lower limbs in patients who retain at least modest motor control.
To date, however, no active fMRI studies were performed in Moyamoya disease. The patient and the NC showed the activation of motor areas, but only the patient showed the ipsilateral motor cortex and cerebellar activation. Cortical activation of not only the lesional hemisphere but also of non-lesional hemisphere is dependent on several factors (e.g., degree of motor function recovery).
It is well established that a high overactivity in nonlesional hemisphere is commonly observed after a cerebrovascular accident. Calautti et al[9] reported that contralesional primary motor area correlated with poor motor performance. These results might explain that activation of the nonlesional area is associated with poor motor performance of the affected upper extremity.
Studies of hand motor function in normal subjects[18,19] have shown that simple hand motor behaviors, such as finger-tapping, could activate the controlateral primary motor cortex (M1) and the cerebellar hemisphere ipsilateral to the movements. Additional areas can be recruited by altering various parameters of movement (complexity, use of the nondominant hand). Such extended networks include the supplementary motor area, the cingulated motor area, the lateral pre-motor-area, the M1 ipsilateral to the movement, and the cerebellum controlateral to the movement. The M1 is highly interconnected with the cerebellar hemisphere on the opposite side of the brain. The cerebellar activation could be related to a direct role in recovery through its postulated role in motor learning and correlate well with recovery after cerebrovascular accident.[18,19] The present data could suggest that activation in the cerebellum areas and in the injured corticospinal tract as well as the activation in ipsi- and controlateral M1 might be related to general recovery processes (even if we performed only one fMRI examination after the neurorehabilitative treatment and none before). The cerebellum, in fact, appears to play an important role in motor recovery after stroke and contains several cognitive-related subregions that are involved in different functional networks implicated in motor and cognitive control. This may facilitate the motor and cognitive recovery in stroke subjects,[20] such as in our patient.
It is possible that as a result of the cerebral revascularization, there may be changes in cortical levels, and hyperperfusion should be seen in motor cortex. However, no data in moyamoya patients were reported about this issue. In addition, the fMRI examination was performed in the chronic phase several months following the neurosurgical intervention and after the rehabilitative training: it is more easy to suppose that the functional activation is the result of the hemodynamic BOLD effects after the motor task, especially if related to cerebellar areas. In fact, the cerebellar circulation depends on basilar artery and not on MCA, and it seems to be related not only to motor performances but also to cognitive performances.
Conclusion
To date, this is the first fMRI case study in a patient affected by moyamoya disease. The case study suggests that fMRI findings might be associated with several factors, such as patient age, family history, and size and location of brain lesions. We believe that our findings provide meaningful information. Further analysis on a will be needed not only to validate our results, but also to suggest the use of fMRI to monitor the motor and the cognitive rehabilitative treatment.
References
1. Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. 2008;7:1056–1066.
2. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009; 360:1226–1237.
3. Baba T, Houkin K, Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry. 2008;79:900–904.
4. Fukui M, Kono S, Sueishi K, et al. Moyamoya disease. Neuropathology. 2000;20:S61–S64.
5. Young AM, Karru SK, Ogulvy CS, et al. Is there a role for treating inflammation in moyamoya disease?: a review of histopathology, genetics, and signaling cascades. Front Neurol. 2013;4:1–5.
6. Karzmark P, Zeifert PD, Bell-Stephens TE, et al. Neurocognitive impairment in adults with moyamoya disease without stroke. Neurosurgery. 2012;70:634–638.
7. Festa JR, Schwarz LR, Pliskin N, et al. Neurocognitive dysfunction in adult moyamoya disease. J Neurol. 2010;257:806–815.
8. Boulos MI, Lena S, Han J, et al. Novel EEG pattern associated with impaired cerebrovascular reserve in Moyamoya disease. Clin Neurophysiol. 2013;125(2):422–425.
9. Calautti C, Jones PS, Naccarato M, et al. The relationship between motor deficit and primary motor cortex hemispheric activation balance after stroke: longitudinal fMRI study. J Neurol Neurosurg Psychiatry. 2010;81:788–792.
10. Bacigaluppi S, Dehdashti AR, Agid R, et al. The contribution of imaging in diagnosis, preoperative assessment, and follow-up of moyamoya disease: a review. Neurosurg Focus. 2009;26:E3.
11. Harada A, Fujii Y, Yoneoka Y, et al. High-field magnetic resonance imaging in patients with moyamoya disease. J Neurosurg. 2001;94:233–237.
12. Yamada I, Nakagawa T, Matsushima Y, et al. High-resolution turbo magnetic resonance angiography for diagnosis of moyamoya disease. Stroke. 2001;32:1825–1831.
13. Conklin J, Fierstra J, Crawley AP, et al. Impaired cerebrovascular reactivity with steal phenomenon is associated with increased diffusion in white matter of patients with moyamoya disease. Stroke. 2010;41:1610–1616.
14. Kuwabara Y, Ichiya Y, Sasaki M, et al. Cerebral hemodynamica and metabolism in moyamoya disease – a positron emission tomography study. Clin Neurol Neurosurg. 1997;99 Suppl 2:S74–78.
15. Wang L, Yu C, Chen H, et al. Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010;133:1224–1238.
16. Grefkes C, Nowak DA, Eickhoff SB, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008:63:236–246.
17. Ward NS, Brown MM, Thompson AJ, et al. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 2003;126:2476–2496.
18. Solodokin A, Hlustik P, Noll DC et al. Lateralization of motor circuits and handedness during finger movements. Eur J Neurol. 2001;8: 425–434.
19. Small SL, Hlustik P, Noll DC, et al. Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain. 2002;125:1544–1557.
20. Li W, Han T, Qin W, et al. Altered connectivity of cognitive-related cerebellar subregions in well-recovered stroke patients. Neural Plasticity. 2013:452439.