 by Saeed Tahmasebi, PhD; Gholamreza Azizi, PhD; Hosein Miladi, MSc; Elham Shafiei, MD; Ali N. Kamali, PhD; Haleh Hamedifar PhD; Farimah Fayyaz, MD; Seyed Erfan Rasouli, BSc; Mostafa Kalvandi, MD; Hamed Mohammadi, PhD; Hadi Hassannia, PhD; Solat Eslami, PhD; Kabir Magaji Hamid, PhD; and Abbas Mirshafiey, PhD
by Saeed Tahmasebi, PhD; Gholamreza Azizi, PhD; Hosein Miladi, MSc; Elham Shafiei, MD; Ali N. Kamali, PhD; Haleh Hamedifar PhD; Farimah Fayyaz, MD; Seyed Erfan Rasouli, BSc; Mostafa Kalvandi, MD; Hamed Mohammadi, PhD; Hadi Hassannia, PhD; Solat Eslami, PhD; Kabir Magaji Hamid, PhD; and Abbas Mirshafiey, PhD
Dr. Tahmasebi is with the Research Center for Applied Plant Sciences, Arak Branch, Islamic Azad University, Arak, Iran. Drs. Azizi and Mohammadi are with the Non-communicable Diseases Research Center at Alborz University of Medical Sciences in Karaj, Iran. Drs. Miladi and Shafie are with the Department of Pathology at the Imam Khomeini Hospital affiliated to Social Security Organization in Arak, Iran. Drs. Kamali and Hamedifar are with CinnaGen Medical Biotechnology Research Center at Alborz University of Medical Sciences in Karaj, Iran and CinnaGen Research and Production Co. in Alborz, Iran. Drs. Fayyaz, Rasouli, and Kalvandi are with the Student Research Committee at Alborz University of Medical Sciences in Karaj, Iran. Dr. Hassannia is with Immunogenetics Research Center, School of Medicine at Mazandaran University of Medical Sciences in Sari, Iran and the Amol Faculty of Paramedical Sciences at Mazandaran University of Medical Sciences in Sari, Iran. Dr. Eslami is with the Dietary Supplements and Probiotic Research Center at Alborz University of Medical Sciences in Karaj, Iran. Dr. Hamid is with the Department of Immunology, School of Medical Laboratory Sciences at Usmanu Danfodiyo University in Sokoto, Nigeria. Dr. Mirshafiey is with the Department of Immunology, School of Public Health at Tehran University of Medical Sciences in Tehran, Iran.
FUNDING: This study was funded by vice chancellor for research, Alborz University of Medical Sciences, under Grant No. 1397-03-00-1829.
DISCLOSURES: The author has no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Recent studies have reported observing antioxidant, anti-inflammatory, and anti-aging properties of α-L-Guluronic acid (G2013) in animal and human studies. It has been theorized that the antioxidant and anti-inflammatory properties of G2013 might be beneficial in epilepsy treatment.
Objective. We sought to determine G2013’s effects on epileptic activity in a kindling-induced animal model.
Methods. Thirty rats were randomly divided evenly into three groups (10 rats in each group): 1) the G2013 group, which was treated with daily injections of G2013 for five days prior to the start of the study; during the 14-day study period, the G2013 rats were given single, daily injections of G2013 that preceded single daily injections of pentylenetetrazole (PTZ), a compound used to induce seizures; 2) the Normal group, which only received injections of saline during the 14-day study, with no seizure induction; and 3) the Control group, which received PTZ injections alone (for seizure induction) for the 14-day study period. The latency between seizure stages and duration of seizures in the G2013 and Control groups were measured using a 5-stage seizure severity scale. Brain samples were taken from all three groups and analyzed histopathologically for parenchymal and meningeal inflammatory cell infiltration. Additionally, the brain samples were analyzed to determine gene expression levels of interleukin-1-beta (IL-1β), IL-6, IL-10), tumor necrosis factor (TNF), chemokine (C-C motif) ligand-2 (CCL2), cyclooxygenase-2 (COX-2), and interferon-gamma (IFN-γ).
Results. The G2013 group demonstrated lower latency between Stages 2 and 5 seizures, with significantly longer mean duration of Stage 5 seizures, compared to the Control group. No significant differences were observed between the three groups histopathologically nor were there any observed differences in gene expression levels.
Conclusion. Our results demonstrated a greater predisposition to PTZ-induced seizures in the rats who received G2013 and PTZ compared to rats who received PTZ alone, suggesting that G2013’s epileptogenic property overshadows its anti-inflammatory effects when applied to a kindled animal model of study.
Keywords: Epileptogenesis, G2013, guluronic acid, pentylenetetrazole, kindling, epilepsy
Innov Clin Neurosci. 2020;17(4–6):9–12
Epilepsy is a neurological disorder that induces abnormal brain activity, causing seizures or episodes of unusual behavior, sensation, and sometimes loss of consciousness.1 The mortality and morbidity of epilepsy varies across countries based on their economic status.2 It is estimated that 65 to 70 million people with epilepsy live in underdeveloped countries, where under-diagnosis, misdiagnosis, and under-treatment are common.3
Multiple factors, including developmental dysfunction, genetic predisposition, and neurological damage—which can cause changes in morphology of synapses and hyperexcitable neuronal transmission—are associated with the development of epilepsy.4 The neuronal damage that can occur from traumatic brain injury, febrile seizures, or hypoxia can predispose an individual to spontaneous recurrent seizures.5
Recent studies have suggested that the inflammatory response might play a pivotal role in epilepsy.1,6,7 Although an inflammatory response in the brain can initiate physiological repair processes,8 deregulation of mediator and receptor expression, caused by the inflammation, can disrupt neuronal activity in the most affected area of the brain.1,9
Recent studies have reported observing antioxidant, anti-inflammatory, and anti-aging properties of the nonsteroidal agent α-L-Guluronic acid (G2013).10-12 Results from preclinical animal studies have suggested that the neuroprotective effects of G2013 observed in rats are due to its ability to reduce nitric oxide (NO) and myeloperoxidase (MPO).10,11 Researchers have also suggested that G2013 can regulate the expression level of the oxidative stress genes,13 and in a clinical study of patients with ankylosing spondylitis or rheumatoid arthritis,14 investigators reported observing antirheumatic effects, including reduced articular and inflammatory symptoms, in the patients who took G2013. These preclinical and clinical data suggest that G2013 might produce beneficial antioxident and anti-inflammatory effects in rat model of epilepsy.
In the current study, we sought to determine G2013’s effects on epileptic activity by measuring seizure latency and duration, as well as changes in brain histopathology and gene expression levels, in seizure-induced rats.
Material and Method
G2013 preparation. To prepare the G2013 (C6 H10O7) compound, a modified method of acid hydrolysis was performed that consisted of dissolving 100g of alginic acid sodium salt (purified) in 20% sulfuric acid (H2SO4) at 0° celsius (C). The solution was blended at room temperature and then warmed to 80°C until it changed color from creamy white to light brown. The hydrolysate was allowed to cool to room temperature, and then was centrifuged at 3,700 × g. The precipitate was dissolved again in 1 mole (M) sodium carbonate Na2CO3, and the pH was adjusted to 2.85 with 0.1M hydrochloric acid (HCl). The solution was then washed using distilled water and placed on petri dishes. The molecular weight and mass of the final substance was confirmed by infrared spectroscopy and carbon-13 nuclear magnetic resonance spectroscopy.13
Animal selection and grouping. This study was approved by the ethical committee of Alborz University of Medical Sciences (IR.ABZUMS.REC.1397.050) and was conducted according to the Helsinki international guidelines on the use of laboratory animals.
Thirty 10-week-old male Wistar rats weighing 250 to 300g were used in the study. The rats were randomly divided into three groups of 10—1) the G2013 group, 2) the Normal group, and 3) the Control group. For adaptation, all rats were kept in cages with 12-hour light-dark cycles for two weeks and were fed the same diet. Rats in the G2013 group received daily injections, intraperitoneally, of G2013 50mg/kg for five days prior to the start of the study. During the 14-day study period, the G2013 group received the same dose of G2013 (50mg/kg) 30 minutes prior to receiving a dose of pentylenetetrazole (PTZ), the compound used to induce seizures. The rats in the Normal group did not receive G2013 before or during the study, and during the 14-day study, these rats only received injections of saline, with no seizure induction (i.e., no injections of PTZ). Rats in the Control group only received PTZ injections for the 14-day study.
Induction of kindled seizures. Kindled seizure was induced using daily intraperitoneal injections of PTZ (37.5mg/kg), diluted with normal saline, for 14 days. After the last injection, the rats were observed for 30 minutes, and were examined for fatality after 30 more minutes. Intensity of seizure was measured using a 1 to 5 stage scale:15 Stage 0=no reaction, Stage 1= vibrissae twitching and hyperactivity, Stage 2=head nodding, Stage 3=unilateral forelimb clonus, Stage 4=bilateral forelimb clonus, and Stage 5=generalized tonic-clonic seizure with loss of postural tone. Rats that received PTZ injections for 14 days and reached three Stage 4 or 5 seizures in a row were considered kindled.
Sample preparation. Following euthanization, the brains of the rats were removed and washed with cool saline. Each brain sample was divided into two lobes. One lobe was stored at -70˚ C for gene expression analysis and the other was stored in 10% formalin for histopathological analysis.
Histopathological assessment. Collected brain samples were divided into sections for histophathological assessment, stained with hematoxylin-eosin (H&E), and examined for parenchymal and meningeal inflammatory cell infiltration. An expert histopathologist analyzed all the prepared sections.
Ribonucleic acid (RNA) analysis for cytokines. For stabilization, purification, and reverse transcription of messenger RNA (mRNA) of the brain tissues to complementary deoxyribonucleic acid (cDNA), RNAlater (Qiagen), RNeasy Lipid Tissue Mini Kits (Qiagen, Hilden, Germany) and Takara kits (Takara, Japan) were used, respectively. Gene expression levels of interleukin-1-beta (IL-1β), IL-6, IL-10, tumor necrosis factor-alpha (TNFα), chemokine (C-C motif) ligand-2 (CCL2), cyclooxygenase-2 (COX-2), and interferon-gamma (IFN-γ) were measured using SYBR Green PCR Master Mix (Takara, Japan) for quantitative reverse transcription polymerase chain reaction (RT-PCR) and were normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) level. The primers used in the setup are shown in Table 1.
Statistical analysis. A one-sample Kolmogorov-Smirnov analysis was used to test whether the variables were normally distributed. Based on the results, nonparametric and parametric analyses were carried out. P-value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 22.0.0.
Results
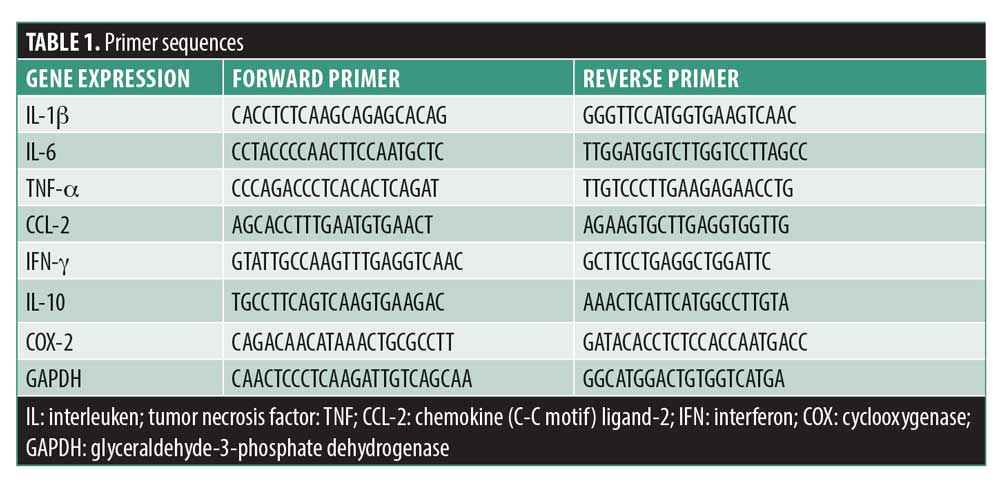
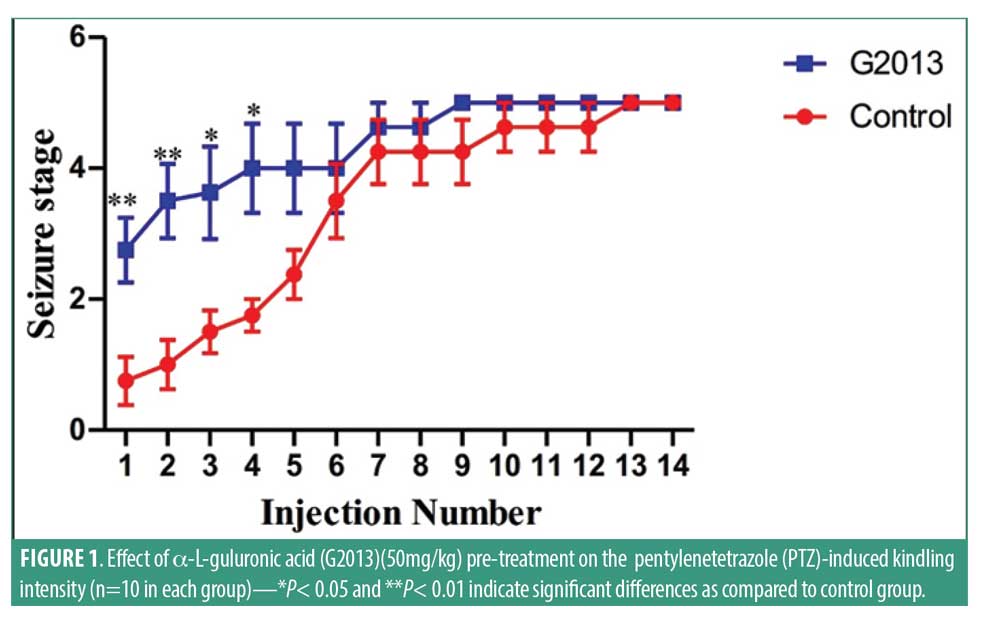
Clinical findings. PTZ-induced kindling intensity. Results indicated that G2013 treatment predisposed the rats in the G2013 group to experience epileptic seizures immediately following the first PTZ injection. G2013 at doses of 50mg/kg increased the duration and intensity of PTZ-induced seizures significantly (Figure 1). The mean stages of seizures reached in the G2013 group were 2.7±1.3, verses 0.8±1.0 in the Control group (p=0.006). Statistical analysis of our findings from first to fourth injection of G2013 indicated significant differences in the seizure intensity between G2013 group and Control group (p<0.05). Additionally, G2013 (50mg/kg) treatment appeared to have a significant effect on the latency between Stage 2 seizures and Stage 5 seizures (Figure 2). The mean (±standard deviation [SD]) latency between Stage 2 and Stage 5 seizures was lower in G2013 group compared to control group (84.8±20.3 vs. 133.0±38.4, p<0.001, and 136.3±41.8 vs. 233.3±142.8, p=0.002), respectively. Moreover, the mean duration of Stage 5 seizures was significantly higher in the G2013 group compared to the Control group (316±69 vs. 197±94, p<0.001) (Figure 3).
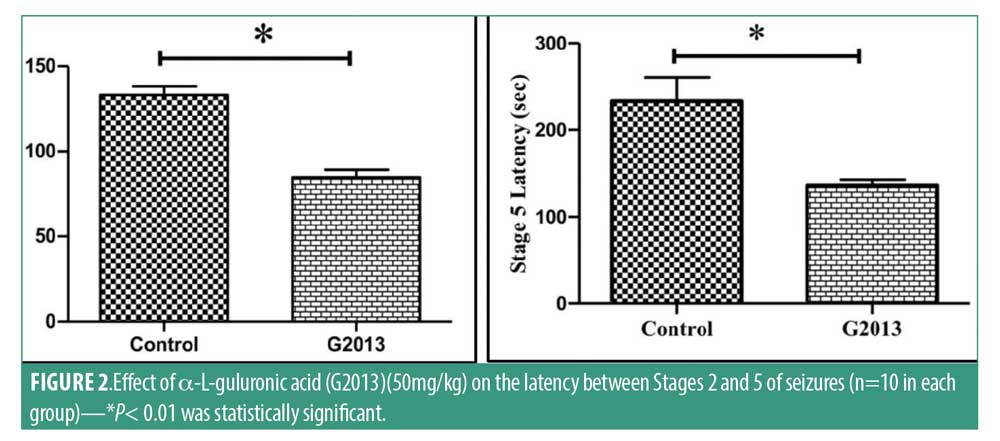
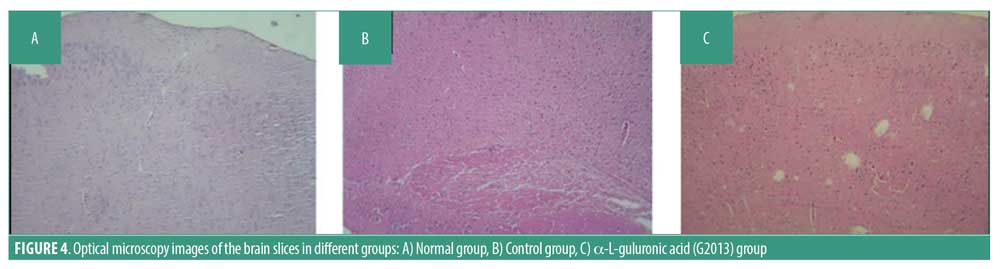
 Histopathological analysis. A minimum of five H&E-stained sections of brain tissue were prepared for each tissue sample in which 10 microscopic fields were examined based on standard criteria in literature. An experienced pathologist assessed the sections using a light microscope, which revealed cystic degeneration, perivascular cuffing congestion, and dead neurons in the G2013 and Control groups, but not in the Normal group (Figure 4).
Histopathological analysis. A minimum of five H&E-stained sections of brain tissue were prepared for each tissue sample in which 10 microscopic fields were examined based on standard criteria in literature. An experienced pathologist assessed the sections using a light microscope, which revealed cystic degeneration, perivascular cuffing congestion, and dead neurons in the G2013 and Control groups, but not in the Normal group (Figure 4).
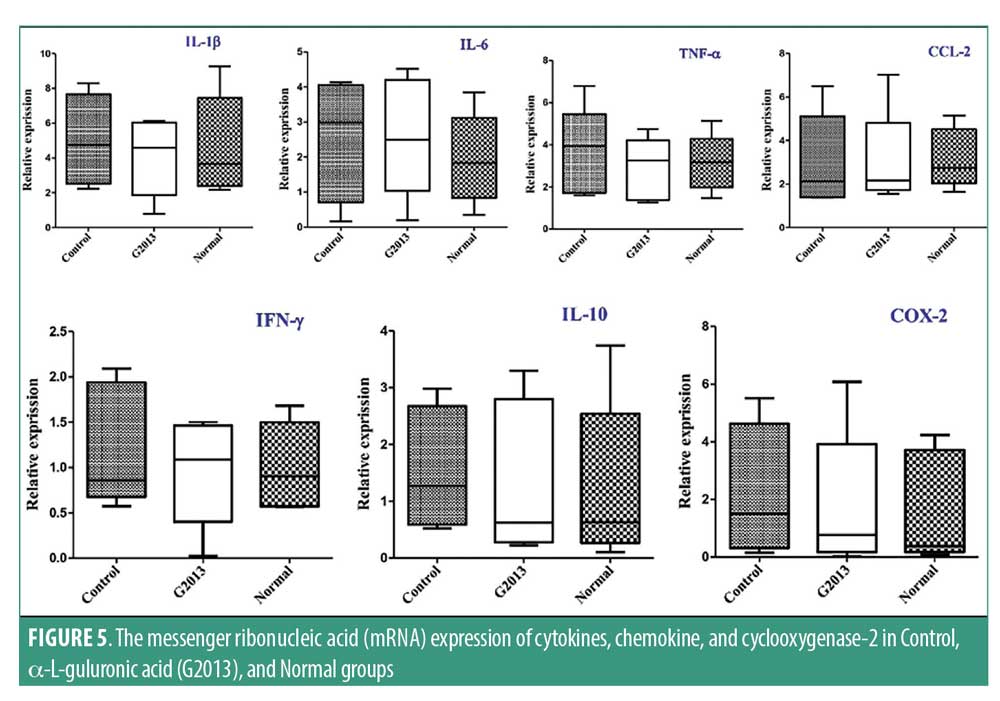
The transcriptional level of cytokines, chemokine and cyclooxygenase-2. The mRNA expression of cytokines, chemokines, and cyclooxygenase-2 genes in the brain tissue was measured by RT-PCR. Expression of IL-6, IL-10, and TNF genes was higher in the Control group compared to the G2013 and Normal groups; however, the differences were not statistically significant. No significant difference was observed in COX-2 expression between all three groups (Figure 5).
Discussion
PTZ administration is a commonly used method for stimulating the central nervous system (CNS) and screening antiepileptic drugs. Chemically induced kindling seizures using PTZ could potentially be used as a model for studying drug-resistant epilepsy and myoclonic and generalized tonic-clonic (primary generalized) seizure. Currently, kindling is considered an important study model for establishing neurochemical and long-term structural changes in the brain caused by epilepsy.16,17 PTZ is an antagonist of GABAA receptor channels and plays a major role in epileptic seizure development.18
In the current study, we observed decreased seizure stage latency, with increased seizure intensity and duration, in the G2013 group compared to the Control group. To our knowledge, this is the first study to use a PTZ-induced seizure model to evaluate the impact of G2013 on GABA receptor channels and glutamate release. We postulate that the mechanism behind G2013’s epileptogenic effect is a disruption in neuronal ion channels and glutamate receptors, and this disruption is potentiated by PTZ’s effect of blocking GABA receptor channels. Additionally, we believe it is possible that G2013 increases PTZ absorption, further increasing the overall epileptogenic effect we observed in our study. Additional research is needed to support our theories.
Previous reports have established the correlation between brain inflammation and epilepsy.7 Research has demonstrated that an inflammatory response in the brain can lead to seizure activity. Some reports have indicated a higher incidence of seizures in patients with chronic inflammatory problems, compared to healthy people.19 Other investigators have theorized that glial cells, triggered by trauma to the brain, play an important role in the pathogenesis of seizures, especially if microglial cells and astrocytes have been activated in response to the injury. Changes in composition and functioning of these cells have been observed in epileptic brain tissues, such as modification of the glutamine/glutamate cycle and alteration of glutamate receptor expression.20-22 The inflammatory process caused by the activation of the glial cells in brain tissue can alter neuronal excitability via stimulation of intracellular signaling pathways, resulting in disruptions of neuron–glial cell interactions. Studies have shown that these changes can lead to neuronal death in the brain that result in seizures.7,23,24
Currently, the investigation of certain molecules that specifically target inflammatory mediators for antiepileptic therapeutic effects is currently underway using animal models.25 Additional research seeking effective methods of halting inflammation in the brain could be useful in developing better, more effective approaches in the prevention and treatment of epilepsy.
Conclusion
G2013 has a low molecular weight and is a natural base agent with anti-inflammatory properties.10-12 G2013 has demonstrated neuroprotective effects (e.g., decreased NO and MPO levels) in preclinical studies using G2013-treated treated mice.10,11 Theoretically, it seems logical that G2013 could inhibit the process of inflammatory diseases by regulation of the expression of GST, iNOS, SOD2, GPX1, CAT, and MPO genes.13 Using a PTZ-induced kindling model, we assessed inflammatory cell infiltration and neuronal death, as well as gene expression levels of inflammatory cytokines (IL-1β, IL-6, TNF-α, IFN-γ, and IL-10), chemokine (CCL-2), and cyclooxygenase-2 (COX-2). Histopathological analysis of brain tissues from G2013-treated rats revealed no differences in inflammatory cell infiltration from the Normal and Control groups, while cystic degeneration, perivascular cuffing congestion, and number of dead neurons were similar between the G2013 and Control groups. Additionally, gene expression levels of IL-1β, IL-6, TNF-α, IFN-γ, and IL-10, CCL-2, and COX-2 were the same in G2013 and Control groups.
Although previous studies have demonstrated anti-inflammatory and immunosuppressive effects of G2013 in the treatment of autoimmune and inflammatory diseases, we observed an epileptogenic effect that masked G2013’s previously reported anti-inflammatory effects, when used in a PTZ- induced kindling model. This suggests that G2013 might not be an effective treatment for epilepsy; it might even excerbate the condition. We theorize that G2013 causes a disruption in the release, degradation, and reabsorption of glutamate that is potentiated by PTZ’s effect of blocking GABA receptor channels, thus resulting the observed epileptogenic effect.
Future research efforts that include behavioral experiments and long-term data are necessary for us to better understand G2013’s epileptogenic effect.
References
- Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. 2018;15(1):144.
- Beghi E, Hesdorffer D. Prevalence of epilepsy–an unknown quantity. Epilepsia. 2014;55(7): 963–967.
- Abramovici S, Bagic A. Epidemiology of epilepsy. Handb Clin Neurol. 2016;138:159–171.
- Rakhade SN, Jensen FE. Epileptogenesis in the immature brain: emerging mechanisms. Nat Rev Neurol. 2009;5(7):380–391.
- Laxer KD, Trinka E, Hirsch LJ, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59–70.
- Vezzani A, Friedman A. Brain inflammation as a biomarker in epilepsy. Biomark Med. 2011; 5(5):607–614.
- Vezzani A, Aronica E, Mazarati A, Pittman QJ. Epilepsy and brain inflammation. Exp Neurol. 2013;244:11–21.
- Chiang N, Serhan CN. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med. 2017;58:114–129.
- Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia.
2005;46(11):1724–1743. - Afraei S et al. New therapeutic approach by G2013 in experimental model of multiple sclerosis. Acta Neurol Belg.
2015;115(3):259–266. - Mirshafiey A, Hosseini S, Afraei S, et al. Anti-aging property of G2013 molecule as a novel immunosuppressive agent on enzymatic and non-enzymatic oxidative stress determinants in Rat model. Curr Drug Discov Techno. 2016;13(1):25–33.
- Hosseini F, Mahdian-Shakib A, Jadidi-Niaragh F, et al. Anti-inflammatory and anti-tumor effects of alpha-l-guluronic acid (G2013) on cancer-related inflammation in a murine breast cancer model. Biomed Pharmacother. 2018;98:793–800.
- Taeb M, Mortazavi-Jahromi SS, Jafarzadeh A, et al. An in vitro evaluation of anti-aging effect of guluronic acid (G2013) based on enzymatic oxidative stress gene expression using healthy individuals PBMCs. Biomed Pharmacother. 2017;90:262–267.
- Mortazavi-Jahromi SS, Nazeri S, Jafarnezhad-Ansariha F,et al. Assessment of immunological profile in ankylosing spondylitis patients following a clinical trial with guluronic acid (G2013), as a new NSAID with immunomodulatory properties. Immunol Res. 2019;67(1):108–115.
- Erakovic V, Zupan G, Varljen J, Simonić A. Pentylenetetrazol-induced seizures and kindling: changes in free fatty acids, superoxide dismutase, and glutathione peroxidase activity. Neurochem Int. 2003;42(2):173–178.
- Ergul Erkec O, Arihan O, Kara M, et al. Effects of Leontice leontopetalum and Bongardia chrysogonum on oxidative stress and neuroprotection in PTZ kindling epilepsy in rats. Cell Mol Biol (Noisy-le-grand). 2018;64(15):71–77.
- Dhir A. Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr Protoc Neurosci. 2012; 58(1):9.37.1–9.37.12
- Rebrov IG, Karpova MN, Andreev AA, et al. Effect of single injection of pentylenetetrazole in a subconvulsive dose on Cl- conductance of the GABAA-receptor complex. Bull Exp Biol Med. 2004;137(1):13–16.
- Vezzani A. Epilepsy and inflammation in the brain: overview and pathophysiology. Epilepsy Curr. 2014;14(1):3–7.
- Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7(3):194–206.
- Vezzani A, Maroso M, Balosso S, et al. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 2011;25(7): 1281–1289.
- Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008; 58(2):168–178.
- Riazi K, Galic MA, Pittman QJ. Contributions of peripheral inflammation to seizure susceptibility: cytokines and brain excitability. Epilepsy Res. 2010;89(1):34–42.
- Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22(6):797–803.
- Dey A, Kang X, Qiu J, Du Y, al Anti-inflammatory small molecules to treat seizures and epilepsy: from bench to bedside. Trends Pharmacol Sci. 2016;37(6):463–484.





