 by Geeta Shroff, MD
by Geeta Shroff, MD
Dr. Shroff is Director of Nutech Mediworld in New Delhi, India.
Innov Clin Neurosci. 2017;14(3–4):12–16.
Funding: No funding was received for the preparation of this article.
Financial disclosures: The author has no conflicts of interest relevant to the content of this article.
Key words: Autism, human embryonic stem cell, hESC, stem cell therapy, neurodevelopmental disorder, brain related disorder
Abstract: Background: Autism spectrum disorder is a neurodevelopmental disorder accompanied by weak immune system and neuroinflammation. Multiple factors contribute to etiology of autism spectrum disorder including genetic disorders, environmental substances/toxins, imbalanced immune system, encephalitis, and viral infections. Autism spectrum disorder is an incurable disease; however, it can be managed by educational and medical interventions. Human embryonic stem cell therapy has been shown to improve blood perfusion in the brain; thus, this therapy may be effective in improving motor skills, social skills, and cognition in patients with autism spectrum disorder.
Method: Three pediatric patients with autism spectrum disorder were administered human embryonic stem cell therapy. Their treatment plan comprised 3 to 4 therapy sessions (T1, T2, T3, T4) that were 4 to 6 weeks in length, with 4- to 8-month gap phases separating each therapy session.
Results: The patients showed improvements in eye coordination, writing, balancing, cognition, and speech and showed reduced hypersensitivity to noises and smells.
Conclusion: The use of human embryonic stem cell therapy may be a safe and effective treatment for patients with autism spectrum disorder. Studies with larger sample sizes are needed to support the use of human embryonic stem cell therapy in this patient population.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by social disconnection, incomplete verbal and nonverbal communication, severely restricted interests, and display of stereotyped and repetitive obsessive behaviors.[1,2] The current diagnosis of ASD is based on the revised criteria in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5).[3] According to the DSM-5, ASD is a single spectrum disorder that comprises three disorders previously described in the fourth edition of the DSM[4] —autism, Asperger’s disorder, and pervasive developmental disorder, not otherwise specified (PDD-NOS). Scientific evidence and clinical practice support that this single spectrum reflects the currently defined symptomatology, time-course, and response to treatment of the disorder.[5]
Multiple factors contribute to the etiology of ASD, including genetic, environmental, immunodeficient, encephalitic, and viral factors.[1] Impairment of methylation and mutations in the mecp2 gene are found to be related to ADS. The genetic polymorphism of cytochrome P450 enzyme, particularly CYP27B1 gene, has also been associated with ASD. CYP27B1 gene is vital for the metabolism of vitamin D, which plays a key role in the neuronal growth and neurodevelopment in the body. Delayed metabolism or deficiency in vitamin D has been reported in the patients with ASD. The other factors deemed responsible for the causation of ASD includes transfer of maternal antibodies, infection in maternal blood, exposure to heavy metals, prenatal folic acid deficiency, genetic disorders, measles, and electromagnetic radiation. These factors can all have detrimental effects on the central nervous system (CNS), neurodevelopment, and environmental responsive genes.[1]
There is no cure for ASD; however, a better quality of life may be achieved by improving intellectual, social, and physical deficits, increasing functional independence, and improving immunity. Stem cell therapy is showing promise in the treatment of incurable diseases. It is believed that stem cell therapy slows or blocks disease progression.[6] Human embryonic stem cells (hESC) are pluripotent, can proliferate indefinitely, and can differentiate into all cell types.[7] This provides a rationale for using hESC therapy in ASD.[8]
We have shown an improvement in patients with cerebral palsy and cortical visual impairment after treatment with hESC therapy.[9] Here, we report the effects of hESC therapy on three pediatric patients with ASD.
Materials and Methods
The present study evaluated the effects of hESC therapy in three patients with ASD. hESCs (NTECH-2000n/nn) are cultured and maintained as per our proprietary, patented technology in a laboratory at Nutech Mediworld (Patent-WO 2007/141657A PCT/1B 2007 Published 13 Dec 2007), which is certified with Good Manufacturing Practices (GTP), Good Laboratory Practices (GLP), and Good Tissue Practices (GTP). The study was approved by an independent institutional ethics committee (IEC) and was conducted in accordance to the principles laid down by “Declaration of Helsinki.”[10] The technique of administrating hESC therapy has been described elsewhere.[9]
The hESC treatment strategy was divided into phases. In the first phase, T1, 0.25ml hESCs ( All patients’ legal guardians provided written, informed consent prior to the treatment. The condition of the patients was videographed before, during, and after the treatment periods. Biochemical and radiological investigations were performed before the start of the treatment and then at regular intervals throughout the treatment phases.
Case Series
Case 1. A three-year-old boy was admitted to Nutech Mediworld on 3 November 2012 with parental complaints of poor attention span, no eye contact, no social interaction, flapping of hands, inability to point at objects, lack of functional independence, mouthing, babbling, and inability to speak clearly.
At the age of one and a half years, the patient’s parents observed that he did not make eye contact, did not respond to his name, and did not play with others. He was diagnosed with ASD at the age of two years, and since then, he has been undergoing occupational and speech therapy.
Patient history revealed that he was born by a lower (uterine) segment caesarean section (LSCS) due to being overdue for delivery (13 days) with no progress of labor.
On investigation, brain single-photon emission computed tomography (SPECT) scan revealed moderate to severe hypoperfusion in bilateral (B/L) frontal and B/L temporal regions. Moderate hypoperfusion was observed in B/L parietal regions and in B/L cerebellar regions.
The patient was treated with hESCs as the primary therapy, along with occupational and physical therapy, for eight weeks starting from 3 November, 2012 to 29 December, 2012. Following the first session of hESC therapy, the patient showed improvements in eye contact, social behaviors, and speaking, and was able to follow basic commands, such as “throw the ball” and “hold the ball.” Mouthing and hand flapping also decreased.
After a gap of four months, the patient returned for the second treatment session. Since his last follow-up visit, his parents reported that he was exhibiting attention-seeking behavior, rocking his body, and throwing tantrums. He followed less commands and had shorter span of eye contact. Following the second treatment session, the patient showed improvement in eye contact, vocabulary, and social awareness. He was playful and became emotionally attached to his parents. The patient also showed improvement in functional independence (e.g., he started wearing sandals on his own).
The brain SPECT scan following the second session showed minimal hypoperfusion in left cerebellar region. Significant improvement in perfusion was seen in the cerebral and cerebellar regions. Figure 1 represents SPECT scan images of the patient before and after the second session of therapy.
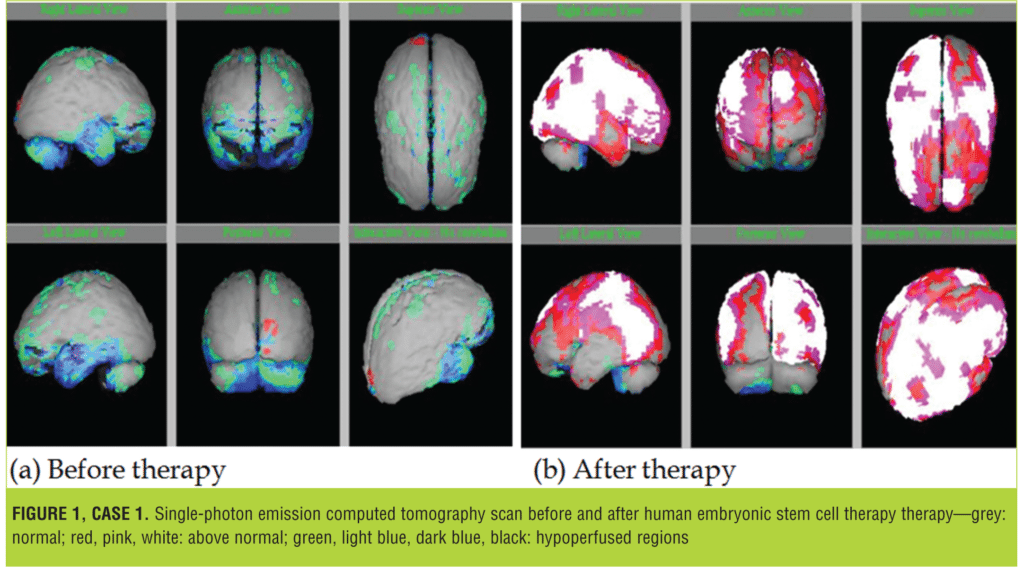
The patient was brought in for the third session of hESC therapy on 28 October, 2013. Following the treatment, improvement was seen in his expressions and attention span, and tantrums and mouthing were significantly reduced, though still present.
Overall, following the three therapy sessions, the patient showed improvement in eye contact and attention span and significant reduction in mouthing. He was able to follow instructions, he showed improvement in functional independence, was playful, and exhibited improved social awareness and emotional attachment to his parents.
Case 2. A 10-year-old boy with impaired speech, uncoordinated talk and walk, low intelligence quotient (IQ), cognitive impairment, and poor concentration was admitted to Nutech Mediworld on 20 June, 2011.
The history revealed that the patient was delivered by induced labor and was forceps assisted. At the age of two years, he exhibited delays in speech, difficulty concentrating, and signs of cognitive impairment. His pediatric neurologist diagnosed him with ASD and pediatric acute-onset neuropsychiatric syndrome. The patient also suffered from respiratory tract infections frequently. Gourmet insertion (B/L) also took place at the age of five years. He suffered from Lyme’s disease syndrome in 2007 for which he underwent the complete Lyme’s treatment protocol with hyperbaric oxygen therapy (HBOT). He also suffered from heavy metal toxicity, which was treated with ethylenediaminetetraacetic acid (EDTA), dimercapto propanesulfonic acid (DMPS), and HBOT.
On examination, the patient had decreased tricep reflex with impaired inarticulated and incoherent speech. He exhibited the signs of impaired sensory perception and obsessive-compulsive disorder (OCD). He was hypersensitive to noises and smells, had weak eye sight, and had night terrors.
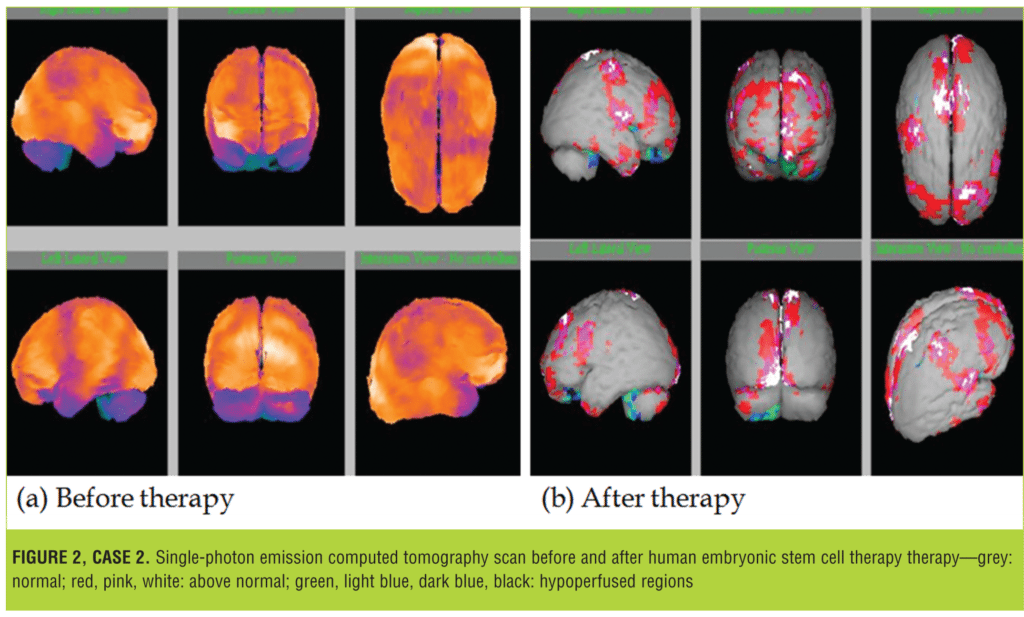
His brain SPECT scan was performed on 21 June 2011, which revealed moderate to severely reduced perfusion in the B/L temporal regions and severe hypoperfusion in the B/L cerebellar regions.
At our facility, the patient underwent hESC therapy as a primary treatment. After receiving four sessions of treatment, there was improvement in his cognition and communication skills. He developed better hand-eye coordination, had improved speech, and could do multiple tasks simultaneously. His hypersensitivity to olfactory/auditory stimulus reduced, he became physically strong, and his immune system improved. After treatment, his SPECT scan, performed 1 July, 2013, revealed normal cerebral perfusion flow. The degree of perfusion observed in the cerebral and cerebellar regions significantly decreased (Figure 2).
Case 3. A four-year-old boy with impaired fine motor and communication skills was admitted to Nutech Mediworld on 27 March, 2012. He exhibited delays in reaching developmental milestones, including speech, flapped his hands, and behaved aggressively.
Prenatally, his mother suffered from gestational diabetes, Hashimoto’s thyroiditis, and alpha thalassemia. The patient was carried to full term and had a normal delivery, but he did not cry spontaneously after birth. He was diagnosed with mild ASD and global developmental delay.
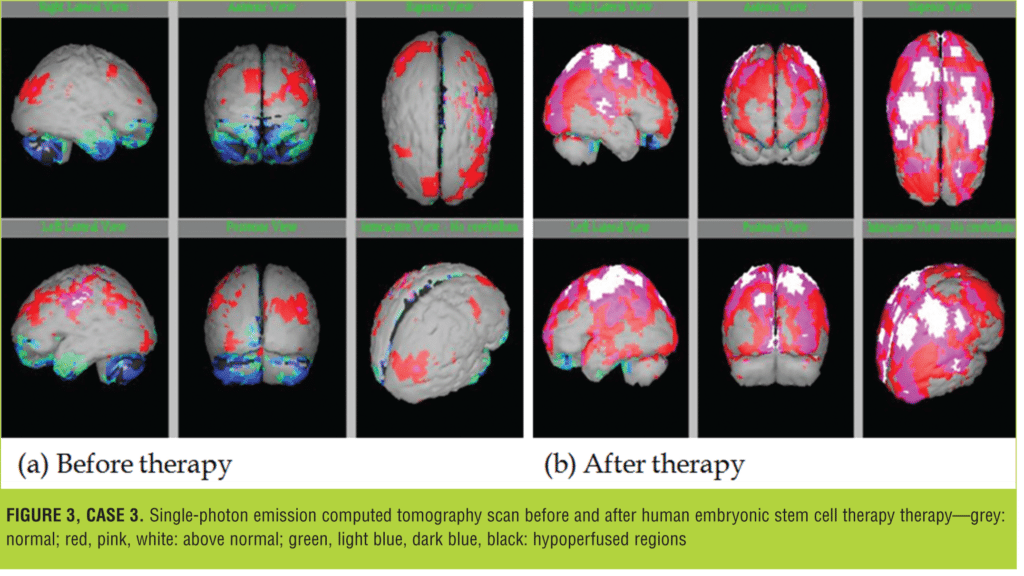
His brain SPECT imaging was performed on 22 March, 2012, which revealed mild-to-moderate hypoperfusion in B/L frontal and temporal and B/L cerebellar regions, respectively.
The patient underwent four sessions of hESC therapy from 27 March, 2012 to 27 September 2014, after which there were improvements in his social interactions, eye contact, intelligence, and motor skills. He could make short sentences and follow commands. As compared to the previous scans, the SPECT scan on 9 January, 2014 revealed normal cerebral perfusion flow (Figure 3).
Discussion
SPECT scans of our three patients showed significant improvement in brain blood perfusion following hESC therapy. We determined this improvement through the use of nuclear imaging—a test used to examine the functioning of organs and the extent of blood perfusion in the brain. SPECT scans are an essential tool for determining cerebral blood flow, cerebral impairment, and local brain metabolism.[11] Moreover, SPECT scans are highly sensitive to detecting abnormalities in the brain.[12,13]
These three pediatric patients with ASD demonstrated significant improvements in communication, writing, and cognitive skills following hESC therapy. Our patients showed improvements in balance and speech, stopped flapping their hands, and were calmer and more physically strong. We also observed a reduction in the inflammation of the eyes of one patient who suffered from eye-related problems.
ASD is a brain-related disorder characterized by an impaired immune system. The most commonly diagnosed pathologies of ASD are impaired CNS circulation, hypoperfusion to the brain, and a weak immune system accompanied by neuroinflammation in the brain.[14,15] It has also been suggested that ASD without a diagnosable cause is a heritable disorder due to its high rate of recurrence among siblings when compared to the general population.[16]
There is no standard approach to treating ASD. In addition to educational and physical therapy interventions, the disorder is often treated with antipsychotic drugs, psychostimulants, and antidepressants.[17] The isolation of hESCs has shown promise in treating various incurable disorders.[18,19] Many recent studies support the use of hESC in treating ASD.[8,15] Studies have also highlighted the use of mesenchymal stem cells (MSCs) in ASD treatment, which can be isolated or generated from hESC.[20] A single registered human clinical trial using MSCs to treat ASD (NCT01343511; http://www.clinicaltrials. gov) aims to test the safety and efficacy of human umbilical cord MSCs and human umbilical cord blood mononuclear cell transplantation in Chinese patients with ASD.[21] The mechanism of action of stem cells in the treatment of ASD might involve the correction of hypoperfusion and immunomodulation.[22] MSCs administered to patients with ASD migrate to the affected region and release survival-promoting growth factors and anti-inflammatory cytokines, which restores plasticity. This mechanism of action might explain the improvements we saw in our study patients after sESC treatment.20 No adverse effects were seen in our patients during the study.
Conclusion
Our patients showed improvement in health, social skills, immunity, and cognition following treatment with hESC therapy. Improvement in brain blood perfusion was reflected in the SPECT scan after hESC therapy. This is the first study reporting the potential role hESCs may have in the treatment of ASD. Studies with larger samples of patients may provide better evidence for the use of hESC more effectively.
Acknowledgment
The author acknowledges all the doctors, staff, and patients of the Nutech Mediworld. The author also acknowledges Knowledge Isotopes Pvt. Ltd. (www.knowledgeisotopes.com) for the editorial support.
References
- Currenti SA. Understanding and determining the etiology of autism. Cell Mol Neurobiol. 2010;30(2):161–171.
- Ratajczak HV. Theoretical aspects of autism: causes–a review. J Immunotoxicol. 2011;8(1):68–79.
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Press Inc.; 2013.
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Press Inc.; 2000.
- Volkmar FR, Reichow B. Autism in DSM-5: progress and challenges. Mol Autism. 2013;4(1):13.
6. - spectrum disorders. The North American Journal of Medicine and Science. 2011;4(3):134–138.
- Yabut O, Bernstein HS. The promise of human embryonic stem cells in aging-associated diseases. Aging (Albany NY). 2011;3(5):494–508.
- Aigner S, Heckel T, Zhang JD, et al. Human pluripotent stem cell models of autism spectrum disorder: emerging frontiers, opportunities, and challenges towards neuronal networks in a dish. Psychopharmacology (Berl). 2014;231(6):1089–1104.
- Shroff G, Gupta A, Barthakur JK. Therapeutic potential of human embryonic stem cell transplantation in patients with cerebral palsy. J Transl Med. 2014;12(1):318.
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Postgrad Med. 2002;48(3):206–208.
- Shroff G, Barthakur JK, Mohan P, Mahajan H. Single photon emission computed tomography scan as a diagnostic tool in children with cerebral palsy treated with human embryonic stem cells. J Nucl Med Radiat Ther. 2015;6(3):1–6.
- Abdel-Dayem HM, Abu-Judeh H, Kumar M, et al. SPECT brain perfusion abnormalities in mild or moderate traumatic brain injury. Clin Nucl Med. 1998;23(5):309–317.
- Abu-Judeh HH, Parker R, Singh M, et al. SPET brain perfusion imaging in mild traumatic brain injury without loss of consciousness and normal computed tomography. Nucl Med Commun. 1999;20(6):505–510.
- Goines P, Van de Water J. The immune system’s role in the biology of autism. Curr Opin Neurol. 2010;23(2):111–117.
- Ng TK, Fortino VR, Pelaez D, Cheung HS. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World J Stem Cells. 2014;6(2):111–119.
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113(5):e472–486.
- Posey DJ, Stigler KA, Erickson CA, McDougle CJ. Antipsychotics in the treatment of autism. J Clin Invest. 2008;118(1):6–14.
- Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2(6):e161.
- Siniscalco D, Bradstreet JJ, Sych N, Antonucci N. Perspectives on the use of stem cells for autism treatment. Stem Cells Int. 2013;2013:262438.
- Siniscalco D, Bradstreet JJ, Sych N, Antonucci N. Mesenchymal stem cells in treating autism: Novel insights. World J Stem Cells. 2014;6(2):173–178.
21. - mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. J Transl Med. 2013;11:196.
- Ichim TE, Solano F, Glenn E, et al. Stem cell therapy for autism. J Transl Med. 2007;5:30.





