by Matteo Berenati, MSc; Antonino Naro, MD, PhD; Cinzia Calabrò, MSc; Michele Torrisi, PsyD; Salvatore Massimiliano Cardali, MD; and Rocco Salvatore Calabrò, MD, PhD
Mr. Berenati, Ms. C. Calabrò, and Drs. Naro, Torrisi, and R. Calabrò are with IRCCS Centro Neurolesi “Bonino-Pulejo” in Messina, Italy. Dr. Cardali is with UOC Neurochirurgia, AOU Policlinico G Martino in Messina, Italy.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
Innov Clin Neurosci. 2021;18(10–12):23–25.
ABSTRACT: Dysarthria refers to a group of disorders resulting from disturbances in neuromuscular control over the speech mechanisms due to damage of the central nervous system (CNS) or peripheral nervous system (PNS). Rehabilitation outcomes of dysarthria significantly depend on the collaboration skills of the patients. This case study aimed to investigate the potential role of neuromuscular electric stimulation (NMES) in improving severe dysarthria. An 18-year-old man affected by severe dysarthria following postanoxic brain injury underwent two different intensive rehabilitation trainings: conventional rehabilitation alone, followed by NMES training alone. We evaluated patient articulation function before and after each training. The overall NMES program was scheduled in daily sessions of 30 minutes, six days a week, for four consecutive weeks. The patient did not report any side effects either during or following both types of intensive rehabilitation training. However, a clear reduction of dysarthria severity was observed only after the NMES training.
Conclusion. NMES could allow for improved articulator expression and vocal parameters, thus enhancing communication skills, when conventional articulation treatments are not possible or are ineffective.
Keywords: Dysarthria, neuromuscular electrical stimulation, severe brain injury, speech therapy
Speech disorders can result from sensorimotor impairments of articulator movements due to different pathologies of the central nervous system (CNS) and peripheral nervous system (PNS), namely dysarthria, or from structural changes of the speech organs, namely dysglossia. Dysarthria is characterized by articulatory-resonatory incompetence, imprecise consonants, distorted vowels, hypernasality, low pitch, harshness, strained/strangled voice, and prosodic disturbances.1 It is often accompanied by deficits in breathing, swallowing, phonation, and resonance.2 The disorder decreases intelligibility of speech, reduces vocal stamina, causes conspicuous voice and manner of speech, and reduces verbal emotional expressivity, with an overall reduction in the quality of life of the patient.3
Intensive functional exercise therapy, including muscle strength training, articulation training, modification of rate or prosody, assistive technology, and/or altering the communication environment, plays a central role in improving articulator impairment. Functional therapy should be based on the pathophysiological underpinnings of dysarthria, including potential disorders of motor control and/or impairment of cognition and behavior.2 Functional therapy may be complemented with prosthetic and surgical approaches and communicational aids in severe cases, including patients with severe acquired brain injury.2 The execution of the conventional rehabilitative treatment for dysarthria is extremely challenging, especially in patients with limited communication skills, including those with disorders of consciousness or other acquired neurological conditions.4 Indeed, limited evidence is available to suggest an immediate beneficial effect of any treatment on impairment level measures, suggesting that people with dysarthria after brain injury will at minimum continue to need rehabilitation according to the current clinical guidelines.4
New instrumental approaches to assist conventional dysarthria treatment are needed. Neuromuscular electric stimulation (NMES) could represent a valid approach. NMES has primarily been used to stregthen muscles, prevent muscle atrophy, and re-educate patients following poststroke dysphagia and central facial palsy.5–8 However, there is insufficient data available on the effects of NMES in dysarthria.
The present case study investigated the effects of NMES in a patient with dysarthria and cognitive impairment resulting from severe postanoxic brain injury. Written informed consent was obtained from the legal guardian for publication of this case report and any accompanying images.
Case Study
An 18-year-old man, who previously underwent cardiac surgery for atrial septal defect, went into cardiac arrest for approximately 15 minutes, resulting in anoxic brain injury. Upon admission to our rehabilitation unit two months later, following the incident the patient was speechless, unable to follow commands, and lacked any eye tracking or limb movement. A diagnosis of unresponsive wakefulness syndrome was made. During hospitalization, he received both pharmacological and physiotherapy treatment. He was tracheostomized and fed with percutaneous endoscopic gastrostomy (PEG). Over the next 16 months, his clinical condition progressively improved to minimally conscious state, and then to conscious wake with severe motor-cognitive disability. Tracheotomy and PEG were removed at that time. It was then evident that the patient had severe speech impairment, with facial weakness, slow and imprecise articulation, vowel distortion, hypernasality, reduced loudness, strained voice quality, and limited pitch variation (AACHENER APHASIE TEST-AAT scores: spontaneous speech: 7, auditory comprehension: 4, repetition: 4, naming: 4).
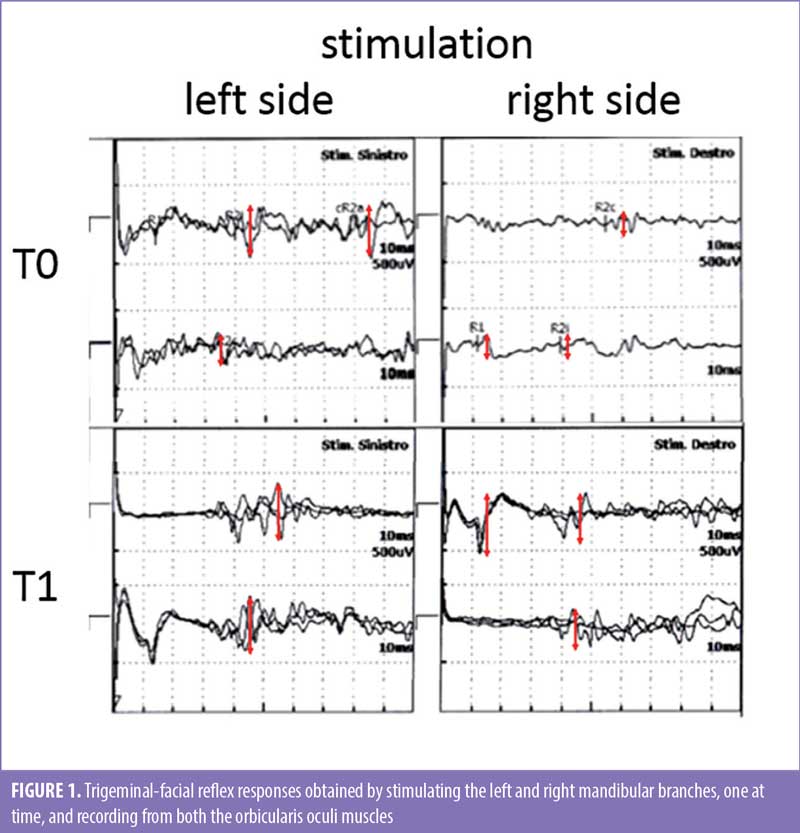
The Aphasia Quotient (AQ), which is the summary score that indicates overall severity of language impairment after Western Aphasia Battery administration, was 28, indicating severe dysarthria. The Robertson Dysarthria Profile (RDP) score was 96. The patient was also tested for trigeminal-facial reflex responses, obtained by stimulating the mandibular branch of the trigeminal nerve with electric pulses and recording electromyographic activity from orbicularis oculi, one side at time, which showed overall low amplitudes and delayed latencies (Figure 1).
A first cycle of conventional speech therapy was ineffective, resulting in minimal changes in dysarthria severity and AQ/RDP scores, due to the patient’s inability to understand instructions and/or carry out orders.
After having obtained informed consent by his legal guardians, the patient was then treated with NMES (VitalStim® Therapy System, DJO; Chattanooga, TN, United States). Because the tool is approved for the treatment of different speech and language pathologies, no ethical issues emerged concerning its application in this patient.
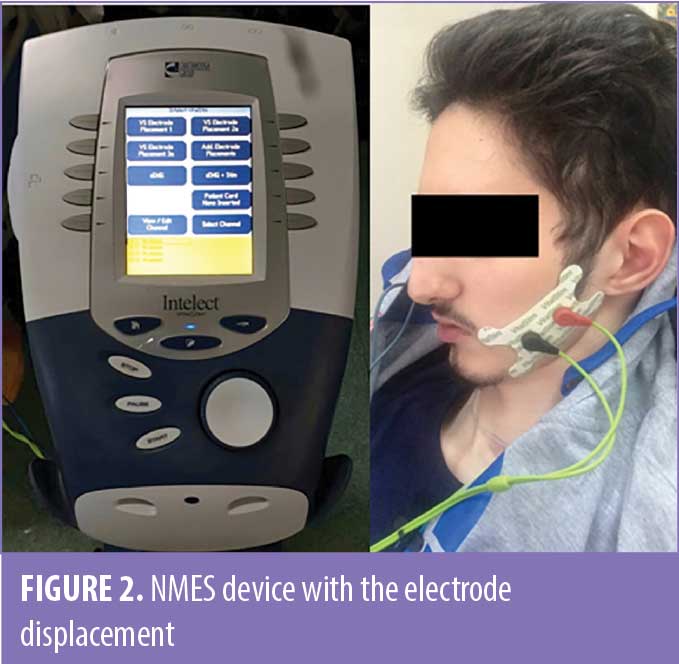
The patient underwent two daily treatment sessions of 30 minutes, six days a week, for four consecutive weeks. Two adhesive electrodes were applied on the facial muscles on both sides of the face, one side at a time, in random order (Figure 2). In this way, we activated the following muscles: masseter (closes the jaw), obicularis oris (purses the lips), risoris (draws the lips into a smile), buccinators (pulls the lips wide and tight), and depressor anguli oris (lowers the bottom corner of the lips). We chose this group of muscles due to its involvement in shaping the sound and air stream into recognizable speech. The stimulation chronaxia was set at 300/260ms, at a perceived intensity of a 3/5 scale, which was well tolerated by the patient, with no observed complications or side effects.
At the end of the NMES treatment, the patient improved 25 to 30 percent in each outcome measure (spontaneous speech: 9, auditory comprehension: 5, repetition: 5, naming: 5, AQ: 36, RDP: 121). Following treatment, the patient showed mild facial weakness, slow and moderately imprecise articulation, vowel distortion, moderate hypernasality, reduced loudness, mildly strained voice quality, and limited pitch variation, with an overall moderate speech impairment severity. There was also a clear increase in amplitudes of trigeminal-facial reflex responses (Figure 1).
Discussion
To the best of our knowledge, this is the first time that NMES was used to treat dysarthria. The sessions took place without concomitant conventional articulation treatments, given that the patient had reduced cognitive skills and was poorly responsive to previous conventional speech therapy. There is only one report on the use of modified NMES-device electroacupuncture to treat dysarthria and a few reports on NMES treatment of patients with neuromotor changes of vocal fold and muscle tension dysphonia.9–10 To date, NMES has been primarily used to treat dysphagia and facial muscle weakness in patients following stroke or other neurological conditions,11 due to the NMES device’s use of specifically designed electrodes that can be applied to the muscles of the throat to promote proper swallow. This NMES treatment has demonstrated effectiveness, though other studies have shown no statistically significant difference between the outcomes of NMES and traditional treatment for dysphagia.12
In our patient, the reduction of dysarthria severity achieved using this NMES device might depend on NMES-induced neuroplastic changes within the sensorimotor areas responsible for articulator functions. NMES and repetitive nerve stimulation have been reported to increase corticobulbar excitability in the long-term,13 suggesting NMES’s ability to reliably drive neuroplasticity and, in turn, give rise to behavioral changes through its effects on brain function. This might be due to a more intensive and repetitive activation of the muscles involved into articulation and the related brain areas.14 The amplitude increase of trigeminal-facial reflex responses suggests a correlation between the neuroplasticity drive and the behavioral changes seen in our patient, possibly resulting in reshaping his cortico-brainstem drive onto the trigeminal motor nucleus. NMES also acts on the peripheral structures beneath the stimulating electrodes (i.e. the motor axons).13,14 The motor units recruited through this pathway discharge relatively synchronously, thus contributing to long-term improvements in neuromuscular function, including muscle strength and fatigue-resistant muscle contractions, functional movements, and muscle quality for training or rehabilitation.14 Notably, the effects on muscle recruitment can vary depending on whether the peripheral nerve trunk or muscle belly has been stimulated.15 In addition, changing the stimulation parameters, which include pulse amplitude, frequency, and duration, might affect the degree of motor axon recruitment and motor unit recruitment order (both temporal and spatial aspects of recruitment) beyond the magnitude of H reflex and other trigeminal-facial reflex responses that are related to central pathway recruitment. Altogether, such issues suggest that NMES can strengthen muscle contractions and improve fatigue resistance, secondarily leading to better muscle activation patterns due to its effects on PNS and CNS.14,15
It is recommended that patients engage in motor exercises during NMES to improve muscle strength, range of movement, coordination, and the biomechanical components involved in articulation, due to the potential lack of specificity in training (i.e., task-oriented). However, our case suggests that NMES motor stimulation without speech exercises can still improve muscle strength, range of movement, and coordination in individuals who exhibit severe dysarthria and are unable to carry out conventional speech therapy.
Conclusion
NMES training shows potential in improving dysarthria in the patients who are unable to carry out conventional speech therapy, thus enhancing communication skills. Considering that no other rehabilitative options are concretely available for this patient population,4 further studies should evaluate the effectiveness of NMES in patients with neurological conditions to enhance our management of dysarthria in fragile, vulnerable, and noncommunicative patients.
References
- Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. J Speech Hear Res. 1969;12(2):246–269.
- Sellars C, Hughes T, Langhorne P. Speech and language therapy for dysarthria due to non-progressive brain damage. Cochrane Database Syst Rev. 2005;(3):CD002088.
- Cohen SM, Elackattu A, Noordzij JP, et al. Palliative treatment of dysphonia and dysarthria. Otolaryngol Clin North Am. 2009;42(1):107–121.
- Mitchell C, Bowen A, Tyson S, et al. Interventions for dysarthria due to stroke and other adult-acquired, non-progressive brain injury. Cochrane Database Syst Rev. 2017;1(1):CD002088.
- Hainaut K, Duchateau J. Neuromuscular electrical stimulation and voluntary exercise. Sports Med. 1992;14(2):100–113.
- Suiter DM, Leder SB, Ruark JL. Effects of neuromuscular electrical stimulation on submental muscle activity. Dysphagia. 2006;21(1):56–60.
- Shaw GY, Sechtem PR, Searl J, et al. Transcutaneous neuromuscular electrical stimulation (VitalStim) curative therapy for severe dysphagia: myth or reality? Ann Otol Rhinol Laryngol. 2007;116(1): 36–44.
- Choi JB. Effect of neuromuscular electrical stimulation on facial muscle strength and oral function in stroke patients with facial palsy. J Phys Ther Sci. 2016;28(9):2541–2543.
- Peng YN, Yin Y, Tan BT. Modified VitalStim electroacupuncture improves the speech function in patients with spastic dysarthria after stroke. Physiotherapy. 2015;101(Suppl 1):e1189.
- Ko KR, Park HJ, Hyun JK, et al. Effect of laryngopharyngeal neuromuscular electrical stimulation on dysphonia accompanied by dysphagia in post-stroke and traumatic brain injury patients: a pilot study. Ann Rehabil Med. 2016;40(4):600–610.
- Tan C, Liu Y, Li W, et al. Transcutaneous neuromuscular electrical stimulation can improve swallowing function in patients with dysphagia caused by non-stroke diseases: a meta-analysis. J Oral Rehabil. 2013;40(6):472–480.
- Permsirivanich W, Tipchatyotin S, Wongchai M, et al. Comparing the effects of rehabilitation swallowing therapy vs. neuromuscular electrical stimulation therapy among stroke patients with persistent pharyngeal dysphagia: a randomized controlled study. J Med Assoc Thai. 2009;92: 259–265
- Calabrò RS, Nibali VC, Naro A, et al. Is non-invasive neuromuscular electrical stimulation effective in severe chronic neurogenic dysphagia? Report on a post-traumatic brain injury patient. NeuroRehabilitation. 2016;38(1):53–57.
- Gondin J, Giannesini B, Vilmen C, et al. Effects of stimulation frequency and pulse duration on fatigue and metabolic cost during a single bout of neuromuscular electrical stimulation. Muscle Nerve. 2010;41(5):667–678.
- Maffiuletti NA. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur J Appl Physiol. 2010;110(2):223–234.





