by Leslie Citrome, MD, MPH; Stephanie S. O’Malley, PhD; David McDonnell, MD; Ying Jiang, PhD; Adam C. Simmons, MPH; Mark P. Berry; and Lauren E. DiPetrillo, PhD
Dr. Citrome is with the Department of Psychiatry and Behavioral Sciences at New York Medical College in Valhalla, New York. Dr. O’Malley is with the Department of Psychiatry at the Yale School of Medicine in New Haven, Connecticut. Dr. McDonnell is with Alkermes Pharma Ireland Limited in Roscommon, Ireland. Drs. Jiang, Simmons, Berry, and DiPetrillo are with Alkermes, Inc. in Waltham, Massachusetts.
Funding: This study was sponsored by Alkermes, Inc., Waltham, Massachusetts, who participated in the design of the study. Funding for editorial support was provided by Alkermes, Inc., Waltham, Massachusetts.
Disclosures: Dr. Citrome was the chair of the independent event adjudication committee for this study and received payment from Alkermes for this role. He also served as consultant and/or advisor to or has received honoraria from Acadia, Alexza, Allergan, AstraZeneca, Avanir, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Forum, Genentech, Intra-Cellular Therapeutics, Janssen, Jazz, Lundbeck, Merck, Medivation, Mylan, Neurocrine, Novartis, Noven, Otsuka, Pfizer, Reckitt Benckiser, Reviva, Shire, Sunovion, Takeda, Teva, Valeant, and Vanda; has stocks in (small number of shares of common stock) Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, and Pfizer (purchased >10 years ago); and received royalties from Wiley (Editor in Chief, International Journal of Clinical Practice), UpToDate (reviewer), and Springer Healthcare (book). Dr. O’Malley reports being a consultant or an advisory board member for Alkermes, Amygdala, Cerecor, Mitsubishi Tanabe, Opiant; a member of the American Society of Clinical Pharmacology Alcohol Clinical Trials Initiative supported by Amygdala, Ethypharm, Lilly, Lundbeck, Otsuka, Pfizer, Arbor Pharmaceuticals, and Indivior. Dr. McDonnell is an employee of Alkermes Pharma Ireland Limited. Drs. Jiang, Simmons, Berry and DiPetrillo are employees of Alkermes, Inc.
Abstract: Background: Alcohol use disorder (AUD) is a common comorbidity in patients with schizophrenia. Although pharmacological options for the management of each disease exist separately, there is no agent approved for both. Moreover, studies conducted in this patient population, who face practical and social challenges as a consequence of being diagnosed with schizophrenia and comorbid AUD, are limited. Methods: We describe the design of a Phase II, double-blind, randomized trial to evaluate adult outpatients with schizophrenia and comorbid AUD receiving a combination of olanzapine plus samidorphan (OLZ+SAM; ALKS 3831), a novel entity currently under development for the treatment of schizophrenia. The combination drug formulation of OLZ+SAM is intended to provide the antipsychotic efficacy of OLZ while mitigating the weight gain and concomitant metabolic abnormalities commonly associated with OLZ alone. In considering this patient population, the novel primary efficacy endpoint is the time from randomization to the first event of exacerbation of disease symptoms (EEDS) based on the occurrence of any of eight prespecified events related to worsening of disease symptoms and/or AUD, as confirmed by a blinded independent adjudication committee. The rate and number of EEDS, improvement in drinking level, and the safety and tolerability of OLZ in combination with SAM will also be assessed. Discussion: A limited number of studies have been conducted in patients with schizophrenia and AUD, and the need for further research in this difficult-to-study population is well documented. This study is, to our knowledge, the largest and longest trial with a randomized, double-blind, active-controlled design. In addition to providing evidence for the development of OLZ+SAM (ALKS 3831) as a therapeutic option, the study aims to provide insights into the clinical management of subjects with schizophrenia and comorbid AUD. Trial registration: Clinical trials NCT02161718, registered May 2014; EudraCT Number: 2014-001211-39.
Keywords: Schizophrenia, alcohol use disorder, ALKS 3831, olanzapine, samidorphan
Innov Clin Neurosci. 2019;16(5–6):15–21
Schizophrenia is a severe and complex psychiatric disorder that has a serious impact on society, affected individuals, and their families.1 The worldwide prevalence of schizophrenia is between 0.5 and 1 percent,2 and the annual incidence is estimated to range from 0.7 to 1.4 per 100,000.3 Alcohol use disorder (AUD) is a common comorbidity among patients with schizophrenia, and the rate of AUD is reported to be generally higher in people with schizophrenia compared to the general population.4 A report from the Epidemiologic Catchment Area study of the United States National Institute of Mental Health estimated that one out of every three people with schizophrenia (33.7%) meet or have met criteria for AUD, which is nearly triple the lifetime prevalence found in the general population (13.5%).4 Schizophrenia with AUD is associated with reduced adherence to treatment,5 increased morbidity, higher levels of inpatient treatment, and violent offending.6
Despite schizophrenia and comorbid AUD representing a distinct and clinically important population in schizophrenia, pharmacological treatments approved for the separate indications have commonly excluded those with both diagnoses, and thus limited clinical trial data are available evaluating pharmacologic interventions in this group.7–9 Some limited literature exists for using clozapine,10 motivational interviewing,11 or long-acting formulations of antipsychotics8 in patients with schizophrenia and AUD. Olanzapine (OLZ) is considered one of the most efficacious antipsychotic medications available for the treatment of schizophrenia12 but has not been studied in prospective controlled trials in patients with comorbid schizophrenia and AUD. OLZ alone has not been effective for primary AUD without schizophrenia.13 The literature supports targeting mu-opioid receptors in the management of brain reward disorders.14,15 Furthermore, additional limited evidence is available for the use of an opioid antagonist in reducing alcohol craving and drinking in patients with schizophrenia and AUD.9,10
Samidorphan (SAM) is a new chemical entity that, in vivo, has been demonstrated to function as a µ-opioid antagonist.16 In vitro, SAM binds with high affinity to human mu-, kappa-, and delta-opioid receptors and acts as an antagonist at mu-opioid receptors and a partial agonist at kappa- and delta-opioid receptors.17 In a Phase II clinical study in subjects with AUD, while SAM did not meet the prespecified endpoint (percentage of subjects abstinent from drinking over 12 weeks), it did significantly reduce the event rate of heavy drinking over that time period, as evidenced in a post-hoc analysis.18
This Phase II, double-blind, randomized trial represents a new pharmacotherapy and unique methodology for assessing efficacy in the treatment of outpatients with schizophrenia and comorbid AUD. The study aims to evaluate a combination of OLZ+SAM (ALKS 3831), a new treatment currently under development for schizophrenia.19 SAM, in combination with OLZ, is hypothesized to confer improved efficacy for schizophrenia with AUD on the occurrence of exacerbation of disease symptoms and provide a favorable safety profile compared to OLZ plus placebo (PBO).
Methods
Study objectives. The primary objective of this study is to evaluate the efficacy of OLZ+SAM compared to OLZ+PBO in adult subjects with schizophrenia and AUD. The primary efficacy endpoint is defined as the time from randomization to the occurrence of an event of exacerbation of disease symptoms (EEDS). An independent event adjudication committee (IAC) will adjudicate whether such events are deemed to be related to the underlying disease, schizophrenia and/or AUD, over a 36- to 60-week double-blind randomized period. This endpoint and study design provide a unique opportunity to evaluate an understudied population of patients with schizophrenia and comorbid AUD.7,20 Secondary efficacy endpoints include the rate and number of EEDS and the proportion of subjects with at least a one-level decrease in World Health Organization (WHO) drinking risk level21,22 from baseline to Week 24 of the double-blind treatment. The latter timepoint was aligned with guidance from the United States Food and Drug Administration (FDA), suggesting that studies for alcoholism treatment are a minimum of six months in duration.23 The safety and tolerability of OLZ in combination with SAM in subjects with schizophrenia and AUD will also be evaluated.
Study design. The study is being conducted across multiple sites in the United States, Bulgaria, and Poland (ClinicalTrial.gov identifier: NCT02161718; EudraCT Number: 2014-001211-39). Following a 30-day screening period, outpatients will participate in a six-week, open-label, lead-in phase, where they will receive open-label OLZ monotherapy once daily for four weeks (dose determined by the investigator). Dose adjustments will be permitted during the first three weeks, as medically required (Figure 1). The OLZ dose will remain fixed for the remainder of the study unless a change is medically indicated. After the first four weeks, open-label OLZ (fixed-dose) combined with SAM (10mg; ALKS 3831) once daily will be administered as separate tablets for two weeks. Subjects who do not tolerate treatment during this two-week period will discontinue, and the remaining subjects will be randomized (1:1) to daily OLZ plus fixed-dose SAM (10mg) or OLZ+PBO for a minimum of 36 weeks and a maximum of 60 weeks. The study will be stopped when the last randomized subject completes his or her 36-week visit after randomization, and all subjects will proceed to a three-week safety follow-up period with open-label OLZ. Thus, the study length allows at least a 36-week period for an EEDS to occur, and the design offers the opportunity for those who enrolled earlier in the study to be followed for up to 60 weeks, allowing for monitoring of recurrent EEDS over time. If withdrawal criteria are not met in subjects referred for EEDS adjudication, they are permitted to continue in the study.
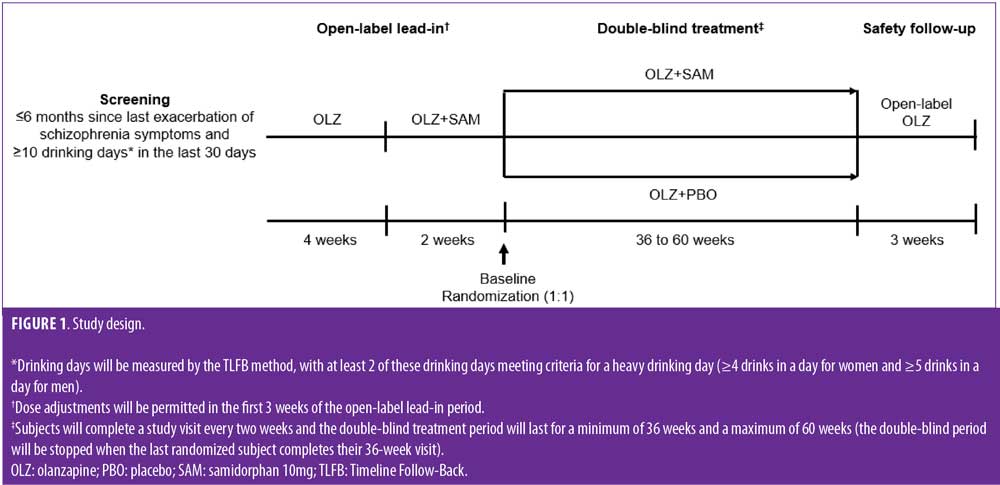
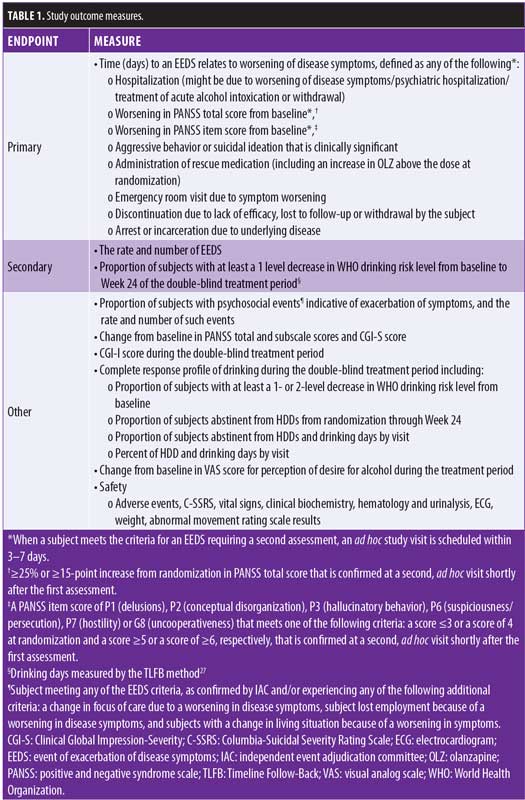
Endpoints and independent adjudication committee. The primary efficacy endpoint is the time from randomization to the first EEDS based on the occurrence, as confirmed by the blinded IAC, of any of eight prespecified events related to worsening of disease symptoms, whether related to schizophrenia or AUD (Table 1). Patients with schizophrenia and AUD have a complex clinical presentation requiring a tailored treatment strategy. Thus, this study uses a primary endpoint specifically intended for this population, combining prospectively defined indicators of symptom recurrence related to significant events, such as hospitalization, emergency room visits, and incarceration. Use of this specific primary endpoint aims to offer a meaningful assessment of the impact of OLZ+SAM on addressing the occurrence of regular exacerbations due to drinking, psychosis, and the combination of both, which can occur in this population.24 Secondary efficacy measures, described in Table 1, include the rate and number of EEDS and the proportion of subjects with at least a one-level decrease in WHO drinking risk level from baseline to Week 24 of the double-blind treatment.
A blinded IAC was established with the purpose of reviewing data from all subjects with a potential EEDS. On initiation of the IAC, all members were required to review a charter detailing the roles and responsibilities of the chairperson and committee members, as well as the protocol to allow for review of the potential cases of EEDS. Meetings are scheduled when a minimum of 20 and maximum of 40 EEDS or subject terminations have occurred. The committee is provided with profiles for potential cases before the IAC meeting. Any supporting information is to be requested from the sponsor as needed. After discussion, voting on whether an EEDS meets protocol criteria for the definition of an EEDS is documented by the IAC chair. The confirmed EEDS events will be used for the efficacy analysis. The IAC comprises an independent group of physicians with experience in clinical management and research in subjects with schizophrenia and comorbid AUD. Members of the IAC are not directly involved in recruitment or trial conduct.
Study participants. Male and female subjects aged 18 to 65 years with a diagnosis of schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, text revision (DSM-IV-TR) criteria25 and a diagnosis of AUD according to DSM Fifth Edition (DSM-5)26 who meet prespecified symptom severity criteria will be eligible for this study. Although DSM-5 was released shortly before the study started, the small differences from DSM-IV-TR schizophrenia criteria were not compelling to address, given the familiarity that investigators already had with the available Mini-International Neuropsychiatric Interview (MINI) instrument used to confirm diagnosis. However, for AUD, the definitions changed considerably and necessitated their adoption to more robustly identify participants with schizophrenia where ALKS 3831 might be of clinical utility. Subjects are required to have experienced a recent acute exacerbation of schizophrenia symptoms (within 6 months), defined as one of the following three criteria: 1) requiring hospitalization (for 7 or fewer days within the previous 30 days); 2) resulting in deliberate self-injury, aggressive behavior, or signs of suicidal or homicidal ideation defined as clinically significant by the investigator; or 3) involving a worsening in symptoms requiring a dose increase or change in antipsychotic treatment or necessitating a visit to an emergency room or an outpatient healthcare facility. The inclusion of subjects experiencing a recent exacerbation of symptoms aims to ensure that exacerbations will be detectable during the study, based on historical documentation and because patterns of exacerbation are hypothesized to be disrupted if alcohol use decreased in the SAM treatment arm. Subjects are required to have a positive and negative syndrome scale (PANSS) total score between 50 and 90, inclusive, and to have a score of four or less for all of the following PANSS Positive (P) Scale or General Psychopathology (G) items: P1 (delusions), P2 (conceptual disorganization), P3 (hallucinatory behavior), P6 (suspiciousness/persecution), P7 (hostility) and G8 (uncooperativeness). Subjects are also required to have a Clinical Global Impression-Severity (CGI-S) score of four or less. Subjects are to have experienced 10 or more drinking days, defined as a day in which the subject had one or more standard alcoholic drinks containing 14g ethanol, in the 30 days prior to screening (as measured by the Timeline Follow-Back [TLFB] method),27 with two or more of these drinking days meeting criteria for a heavy drinking day (defined as four drinks in a day for women and five drinks in a day for men).
Key exclusion criteria include an intolerance to OLZ, a positive test for opioids, having taken opioid agonists within 14 days prior to screening or anticipating the need to take such medication during the study, and having taken opioid antagonists within 60 days prior to screening. Substance use disorders other than AUD within the past one year, as defined by DSM-5, are excluded. Additional exclusion criteria includes first psychotic episode one year or less before screening or only a single lifetime psychotic episode, and presence of any other medical or psychiatric condition that could impact safety or complicate treatment.
The study protocol and informed consent form were approved by an Institutional Review Board/Independent Ethics Committee at each study site prior to subject recruitment. The study will be conducted in accordance with the International Conference on Harmonization Good Clinical Practices guidelines and in line with the ethical principles of the Declaration of Helsinki. All study participants are to review and sign an informed consent document explaining study procedures and potential risks before study entry.
Treatments. The oral treatments are to be administered once daily at the same time, as close to bedtime as possible. Subjects not receiving antipsychotic medication at screening will be titrated to OLZ at the beginning of the open-label period. Subjects taking an antipsychotic other than OLZ will be tapered off medication and titrated to OLZ at the beginning of the open-label period. The process of transitioning from another antipsychotic is to be individualized based on clinician judgment. Subjects already receiving OLZ at screening will continue with treatment as prescribed for a period of four weeks prior to initiation of OLZ+SAM. Following randomization, subjects will enter the double-blind period and will receive their allocated treatment (OLZ+SAM or OLZ+PBO); PBO and SAM tablets are identical in size and appearance. Following randomization, a decrease in OLZ dose is permitted when medically indicated (e.g., if the subject has stable disease but is experiencing adverse events). An increase above the OLZ dose level allocated at randomization will be considered as a possible EEDS and will be referred to the IAC for adjudication.
Clinical assessments and evaluations. During the double-blind treatment period, which is a minimum of 36 weeks and a maximum of 60 weeks, an outpatient visit is scheduled every two weeks. Each visit involves clinical assessments plus drug dispensing (every 4 weeks) or drug dispensing only (every 2 weeks). The initial two clinical assessment visits after randomization are being conducted at two-week intervals to allow for initial safety monitoring. Adverse events (AEs) will be monitored continuously from when informed consent is obtained until the final study visit. Subjects discontinuing treatment or withdrawing from the study early will be asked to return for an early termination visit, where the procedures scheduled for the final study visit can be conducted and the safety follow-up can be completed. When a subject meets the criteria for an EEDS requiring a second assessment, an ad hoc study visit will be scheduled within 3 to 7 days (Table 1). If, despite all reasonable efforts, a second assessment cannot be completed within seven days, the event will be considered an EEDS for adjudication by the IAC. If no withdrawal criteria are met following adjudication, subjects experiencing an EEDS (except discontinuation due to lack of efficacy/lost to follow-up/withdrawal by subject) will be permitted to continue in the study.
Schizophrenia symptom assessments include the Positive and Negative Syndrome Scale (PANSS)28 and Clinical Global Impression – Severity (CGI-S)29 scales. Alcohol drinking is to be assessed using the TLFB method.27 At the screening visit, data on alcohol use will be collected for the prior 30-day period. At all other visits, data will be collected for the period between the last completed TLFB assessment and the current visit. The subject is to be assisted by the same person each time he or she is asked to complete the TLFB. Heavy drinking days (HDDs) are defined as at least four drinks in a day for female patients and five drinks in a day for male patients, with a standard drink containing 14g ethanol. To determine WHO drinking risk level, drinks per day in grams is computed according to the following equation: total number of standard drinks x 14 grams (g)/total number of days in the period. Drinks per day in grams is then classified into risk levels as follows: abstinence (0g); low risk (male patients 1–40g, female patients 1–20g); medium risk (male patients 41–60g, female patients 21–40g); high risk (male patients 61–100g, female patients 41–60g); very high risk (male patients 101g or greater, female patients 61g or greater).21,22 For the assessment of desire for alcohol, subjects are requested to complete a visual analog scale (VAS) ranging from “no desire at all for alcohol”(marked with a “0”) to “strongest imaginable desire for alcohol” (marked with a “100”).
Statistical analyses. Sample size determination is based on the detection of log-hazard ratio for the first EEDS during the double-blind treatment period. Based on the previous clinical studies with OLZ and clinical judgment, the cumulative proportion of EEDS by the end of a 36-week double-blind treatment period is estimated as 15 and 30 percent for the OLZ+SAM group and the OLZ+PBO group, respectively. Assuming an exponential distribution of survival time and a two-sided test at alpha=0.05, a total of 70 recurrent events are required for approximately 90 percent power to detect a hazard ratio of 0.45 between OLZ+SAM and OLZ+PBO, with a randomization allocation ratio of 1:1 (OLZ+SAM:OLZ+PBO). It is estimated that approximately 270 (135 subjects per treatment group) will be randomized to the double-blind treatment phase to allow observation of 70 EEDS.
The efficacy analyses will be carried out using the intention-to-treat (ITT) population, defined as all randomized subjects who receive at least one dose of their assigned treatment. The primary efficacy endpoint is defined as the time (days) from randomization to an EEDS. For the primary analysis, event rate will be estimated by the Kaplan-Meier method. The log-rank test will be used for treatment comparison, and the Cox proportional-hazards model will be used to estimate the hazard ratio, adjusting for relevant covariates. Regarding analysis of secondary efficacy endpoints, the time (days) to recurrent EEDS will be analyzed by an Andersen-Gill model, and the proportion of subjects with at least a one-level improvement in WHO drinking risk level at Week 24 of the double-blind treatment period will be compared between groups using a logistic regression model based on last observation carried forward imputation for the missing data. Safety will be assessed using descriptive statistics.
Discussion
Here, we describe the protocol and study design for a Phase II, multicenter, randomized, double-blind, placebo-controlled study evaluating the efficacy, safety, and tolerability of daily oral OLZ+SAM (ALKS 3831) compared to OLZ+PBO in adults with schizophrenia and comorbid AUD. It is hypothesized that subjects receiving treatment with OLZ+SAM will drink less alcohol, helping them to maintain symptom control with OLZ and thus experience a lower rate of EEDS, as well as a favorable safety profile compared to OLZ+PBO.
An overview of studies previously conducted in patients with schizophrenia and AUD is provided in Table 2. The observation that a limited number of studies have been conducted in this population has been documented by a number of authors.7,8,20 Guidelines and treatment algorithms for patients with schizophrenia have generally been based on studies conducted in noncomorbid populations,7 a deficiency that needs to be addressed. To this point, there is a need for further research involving well-designed, controlled studies in such an understudied population. Such research will help determine strategies for clinical management.8,20

In general, the previous studies presented in Table 2 have involved low patient numbers (approximately 30 or fewer) and have been of relatively short duration (generally a maximum of 12 weeks long), with the exception of one six-month study that included 95 patients8 and a retrospective review that included 86 cases.30 The authors highlighted the challenges with conducting clinical trials in a comorbid population, and they acknowledged the difficulties of recruiting sufficient participants to ensure an adequate sample size for statistical analysis.8 It is well known that patients with schizophrenia and AUD offer unique study challenges,31 typically being difficult to recruit and often presenting with other obstacles that might hinder the ability to actively engage them and maximize retention in an interventional study, (e.g., cognitive impairment, lack of insight).20 The present study aims to randomize approximately 270 individuals, potentially making this one of the largest studies conducted in this population. Furthermore, the double-blind treatment period of 36 to 60 weeks is longer than any previous study, allowing for the monitoring of recurrent EEDS over time. Considering the challenges of implementing a clinical trial in this population, this study is, to our knowledge, the largest and longest study with a randomized, double-blind design where all subjects receive the same antipsychotic medication (OLZ) in combination with either SAM or PBO.
Designing a study to provide meaningful clinical insights in a patient population with a complex diagnosis presented a challenge. The difficulty in evaluating a link between alcohol drinking and symptom worsening in this patient population has been acknowledged.7 Since commonly used endpoints for psychosis and alcohol drinking might not adequately evaluate the benefit for subjects presenting with both conditions combined, this study aims to use an endpoint specific to this population. This endpoint combines indicators of symptom recurrence related to significant events for those with schizophrenia and AUD, such as hospitalization, emergency room visits, and aggressive behavior. The primary endpoint aims to offer an assessment of the impact of OLZ+SAM on a variety of clinically meaningful outcomes. In addition, the collection of longitudinal data on drinking, over the 36-to-60-week, double-blind, randomized period, aims to elucidate the role of alcohol consumption in exacerbation of disease symptoms in this subject population.
Findings from this study, using a novel endpoint and a unique study design, will offer an opportunity to expand the current research and provide further insights into treatment and clinical management of this understudied population. Furthermore, this research will provide guidance and insights to the scientific community to assist in the design of future studies in this subject population.
Limitations. There are some potential limitations for consideration. Findings from this study may be confounded by the lead-in period, as this may exclude the highest risk of relapse patients and also bias the patients to those those who respond to olanzapine. In addition, the variable length of exposure to treatment after Week 36 may limit the interpretation of findings during the 36–60-week period.
Acknowledgments
The authors would like to thank Mark S. Todtenkopf, PhD, who assisted in the preparation and proofreading of the manuscript. Medical writing and editorial support for the preparation of this manuscript (under the guidance of the authors) was provided by Louise Brady, PhD, (ApotheCom, UK).
Author Contributions
Dr. Citrome is the IAC Chairperson. Dr. O’Malley contributed to the design of the study and the analysis plan related to alcohol endpoints. Dr. McDonnell was involved in the study design and planning and is the medical monitor for the study. Dr. Jiang was involved in development of the statistical analysis plan. Mr. Simmons was involved in the study design and planning, including development of the statistical analysis plan, investigator training, and ongoing data review and interpretation. Mr. Berry is the clinical trial manager involved with day-to-day management of the study. Dr. DiPetrillo was involved in the study design and planning, including development of the statistical analysis plan, investigator training, and ongoing data review and interpretation. All authors were involved in writing the manuscript and read and approved the final version prior to submission.
References
- Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97.
- Centers for Disease Control and Prevention (CDC). Burden of Mental Illness. Schizophrenia. 2013. https://www.cdc.gov/mentalhealth/basics/burden.htm. Accessed Sept. 1, 2018.
- Jablensky A, Sartorius N, Ernberg G, et al. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr Suppl. 1992;20:1–97.
- Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–2518.
- Werner FM, Covenas R. Long-term administration of antipsychotic drugs in schizophrenia and influence of substance and drug abuse on the disease outcome. Curr Drug Abuse Rev. 2017;10(1):19–24.
- Jones RM, Lichtenstein P, Grann M, et al. Alcohol use disorders in schizophrenia: a national cohort study of 12,653 patients. J Clin Psychiatry. 2011;72(6):775–779; quiz 878–879.
- Petrakis IL. How to best treat patients with schizophrenia and co-occurring alcohol use disorder. J Clin Psychiatry. 2015;76(10):e1338–1339.
- Green AI, Brunette MF, Dawson R, et al. Long-acting injectable vs oral risperidone for schizophrenia and co-occurring alcohol use disorder: a randomized trial. J Clin Psychiatry. 2015;76(10):1359–1365.
- Petrakis IL, O’Malley S, Rounsaville B, et al. Naltrexone augmentation of neuroleptic treatment in alcohol abusing patients with schizophrenia. Psychopharmacology. 2004;172(3):291–297.
- Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia. Part 3: update 2015 management of special circumstances: depression, suicidality, substance use disorders and pregnancy and lactation. World J Biol Psychiatry. 2015;16(3):142–170.
- De Witte NA, Crunelle CL, Sabbe B, et al. Treatment for outpatients with comorbid schizophrenia and substance use disorders: a review. Eur Addict Res. 2014;20(3):105–114.
- Citrome L. A systematic review of meta-analyses of the efficacy of oral atypical antipsychotics for the treatment of adult patients with schizophrenia. Expert Opin Pharmacother. 2012;13(11):1545–1573.
- Kishi T, Sevy S, Chekuri R, Correll CU. Antipsychotics for primary alcohol dependence: a systematic review and meta-analysis of placebo-controlled trials. J Clin Psychiatry. 2013;74(7):e642–654.
- Petrakis IL, Nich C, Ralevski E. Psychotic spectrum disorders and alcohol abuse: a review of pharmacotherapeutic strategies and a report on the effectiveness of naltrexone and disulfiram. Schizophr Bull. 2006;32(4):644–654.
- Barrio P, Ortega L, Guardia J, et al. Who receives nalmefene and how does it work in the real world? A single-arm, Phase IV study of nalmefene in alcohol dependent outpatients: baseline and 1-month results. Clin Drug Investig. 2018;38(2):147–155.
- Shram MJ, Silverman B, Ehrich E, et al. Use of remifentanil in a novel clinical paradigm to characterize onset and duration of opioid blockade by samidorphan, a potent mu-receptor antagonist. J Clin Psychopharmacol. 2015;35(3):242–249.
- Bidlack JM, Knapp BI, Deaver DR, et al. In vitro pharmacological characterization of buprenorphine, samidorphan, and combinations being developed as an adjunctive treatment of major depressive disorder. J Pharmacol Exp Ther. 2018;367:267–281.
- Silverman BL, Kane JM, Todtenkopf MS, et al. Early development of ALKS 3831: a novel drug candidate for the treatment of schizophrenia. In: New Clinical Evaluation Drug Unit Annual Meeting. Hollywood, Florida USA; 2013.
- Silverman BL, Martin W, Memisoglu A, et al. A randomized, double-blind, placebo-controlled proof of concept study to evaluate samidorphan in the prevention of olanzapine-induced weight gain in healthy volunteers. Schizophr Res. 2018;195:245–251.
- Sawicka M, Tracy DK. Naltrexone efficacy in treating alcohol-use disorder in individuals with comorbid psychosis: a systematic review. Ther Adv Psychopharmacol. 2017;7(8-9):211–224.
- Witkiewitz K, Hallgren KA, Kranzler HR, et al. Clinical validation of reduced alcohol consumption after treatment for alcohol dependence using the World Health Organization risk drinking levels. Alcohol Clin Exp Res. 2017;41(1):179–186.
- World Health Organization. Dept. of Mental Health and Substance Dependence. (2000). International guide for monitoring alcohol consumption and related harm. Geneva: World Health Organization.
- U.S. Department of Health and Human Services (DHHS), Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Alcoholism: developing drugs for treatment. Guidance for Industry. February 2015. https://www.fda.gov/files/drugs/published/Alcoholism—Developing-Drugs-for-Treatment.pdf. Accessed January 1, 2019.
- Gerding LB, Labbate LA, Measom MO, et al. Alcohol dependence and hospitalization in schizophrenia. Schizophr Res. 1999;38(1):71–75.
- Association Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th Ed., text revision (DSM-IV-TR). Washington, DC. American Psychiatric Association. 2000.
- Association Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-5). Arlington, VA. American Psychiatric Assocation. 2013.
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. Litten RZ, Allen JP (Eds). In: Measuring alcohol consumption: Psychological and biological methods. Totowa, NJ: The Humana Press; 1992:41–72.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276.
- Guy W. Clinical Global Impressions (CGI) Scale. In: Handbook of Psychiatric Measures. Edited by Rush JR, First MB, Blacker D, Endicott, Keith SJ, Ryan ND, Smith GR, Tsuang MT, Widiger TA, Zarin DA. APA, Washington, DC; 2000:100–102.
- Brunette MF, O’Keefe C, Zimmet S, et al. Clozapine, olanzapine, or typical antipsychotics for alcohol use disorder in patients with schizophrenia. J Dual Diagnosis. 2008;4(4):344–354.
- Ziedonis DM, Smelson D, Rosenthal RN, et al. Improving the care of individuals with schizophrenia and substance use disorders: consensus recommendations. J Psychiatr Pract. 2005;11(5):315–339.
- Feeley RJ, Arnaout B, Yoon G. Effective switch from clozapine to aripiprazole in treatment-resistant schizophrenia and comorbid alcohol use disorder. J Clin Psychopharmacol. 2017;37(6):729–730.
- Ralevski E, O’Brien E, Jane JS, et al. Treatment with acamprosate in patients with schizophrenia spectrum disorders and comorbid alcohol dependence. J Dual Diagnosis. 2011;7(1-2):64–73.
- Batki SL, Dimmock JA, Wade M, et al. Monitored naltrexone without counseling for alcohol abuse/dependence in schizophrenia-spectrum disorders. Am J Addict. 2007;16(4):253–259.



