
by David G. Daniel, MD; Noah G. Daniel; Donald T. Daniel; Laura Copeland Flynn, MS; and Michael H. Allen, MD
Dr. Daniel is with Bioniche Global Development, LLC in McLean, Virginia and the George Washington University in Washington DC. Mr. N. Daniel is with Dartmouth College in Hanover, New Hampshire. Mr. D. Daniel is with Brown University in Providence, Rhode Island. Ms. Flynn is with LCF Consulting, LLC in Lake Forest, Illinois. Dr. Allen is with the University of Colorado School of Medicine in Aurora, Colorado.
Funding: Support for this study was provided by Bioniche Global Development, LLC.
Disclosures: Dr. Daniel is the president of Bioniche Global Development, LLC, which has an intellectual property interest in propofol for the rapid treatment of depression and suicidality. Dr. Allen, Ms. Copeland Flynn, Noah Daniel and Donald Daniel are consultants to Bioniche Development.
Abstract: Objective: Propofol (2,6-diisopropylphenol) is a gamma-aminobutyric acid type A agonist intravenous anesthetic agent used in outpatient settings. Based on anecdotal reports of improved mood in humans following propofol-induced anesthesia, the impact of acute propofol treatment alone or in combination with subchronic fluoxetine dosing was tested on forced swim test (FST) performance. Design: Seventy-two adult male mice (C57/BL6, CRL-provided) were pretreated daily with saline or fluoxetine (20 mg/kg, intraperitoneally) (21 days for cohort 1; 24 days for cohort 2). At 24 hours after the last pretreatment injection, the mice received saline or propofol (35 or 50 mg/kg, intraperitoneally). Then, 45 minutes later, the mice underwent a five-minute FST. Immobility time was quantified and evaluated with a custom video-analysis software program. Results: A one-way analysis of variance indicated statistically significant effects of propofol on immobility time in cohorts 1 and 2. A comparison performed using Dunnett’s method revealed that propofol 50 mg/kg (p < 0.05) but not 35 mg/kg (p = not significant) reduced immobility time as compared with in the saline–saline control group (difference between means of 38.42 and 16.46 seconds, respectively). Conclusion: In comparison with saline, propofol significantly decreased immobility time during the FST, which models depression and resilience to stress. Our preclinical results are consistent with a small open-label study of propofol used in treatment-resistant depression recently reported by Mickey BJ, White AT, Arp AM, et al (2018). Further investigation of propofol regarding its potential antidepressant effects seems warranted.
Keywords: Forced swim test, mouse model, propofol, treatment-resistant depression
Innov Clin Neurosci. 2019;16(9–10):22–26
In 2017, the World Health Organization estimated that depressive illness was affecting more than 300 million people.1 The prevalence of treatment-resistant depression (TRD) is estimated to be 15 to 30 percent of all major depression cases. TRD entails increased disability and risk of suicidal behavior.2,3 Dysfunction of glutaminergic systems is thought to play a role in the pathophysiology of TRD. Ketamine, esketamine, rapastinel, and other investigational agents with varying properties of N-methyl-D-aspartate receptor antagonism have produced rapid-onset antidepressant-like effects in rodent models of depression and in depressed patients.4–7 Potential limitations of ketamine include increases in blood pressure, nausea, dizziness, blurred vision, and psychomimetic effects.8 Esketamine (Spravato) was recently approved by the US Food and Drug Administration in combination with an orally administered antidepressant for treatment resistant depression. Brexanolone, which modulates gamma-aminobutyric acid (GABA), has a rapid onset of action, is administered intravenously, and was recently approved for treatment of postpartum depression. Rapstinel recently failed to significantly separate from placebo in three studies assessing its utility as an adjunctive treatment for depression.
Propofol (2,6-diisopropylphenol) is a gamma-aminobutyric acid type A (GABA-A) agonist intravenous anesthetic agent in use since 1986. Propofol is commonly administered in ambulatory settings because of its rapid onset, dose-related hypnotic effect, rapid recovery, and favorable safety profile.9–11 It is typically formulated in an oil-in-water emulsion and is highly lipophilic, with a rapid onset and short duration of action.12 Propofol’s anesthetic effects are felt to derive principally from modulating the inhibitory function of the neurotransmitter GABA through GABA-A receptors.9 However, propofol also inhibits N-methyl-D-aspartate receptor (NMDA)receptors (although it is unclear whether this occurs appreciably at clinically relevant doses), reduces calcium influx through slow calcium channels, and possesses antioxidant, immunomodulatory, and anticonvulsant activity.11,13,14 In addition, propofol inhibits acetylcholine release from the frontal cortex and hippocampus.11,15
Based on anecdotal reports of improved mood in humans following propofol-induced anesthesia, we tested acute propofol treatment alone and in combination with subchronic fluoxetine dosing on forced swim test (FST) performance. The FST is a commonly applied animal model of depression in which R-ketamine and rapastinel have previously demonstrated rapid antidepressant effects.16–18 If there was sufficient preclinical evidence of antidepressant potential to investigate propofol clinically, substantial human pharmacokinetic and safety information would be available as a background for clinical testing.
Methods
The objective of this study was to evaluate the effects of acute propofol treatment alone or in combination with subchronic (21 or 24 days) fluoxetine dosing on forced swim test (FST) performance. To do so, two separate cohorts of mice were daily pretreated with saline or fluoxetine (20mg/kg, intraperitoneally) (21 days for Cohort 1; 24 days for Cohort 2). At 24 hours after the last pretreatment injection, the mice received either saline or propofol (35 or 50mg/kg, intraperitoneally) treatment. Forty-five minutes later, the mice underwent a five-minute FST, with their time spent immobile (i.e., floating behavior) quantified.
Test facility and test site. The biotechnical experiments described in the present report were performed at Charles River Laboratories (CRL; San Francisco, California). The animal experiments were conducted under the supervision of Drs. Nadege Morisot and Holden Brown Janssens, who completed the study report on September 27, 2018.19
Archiving. All raw data are located in the archive of CRL under Study Number Key 1680. Data will be stored for a period of 10 years after completion of the final report.
Animals. Seventy-two adult male mice (C57/BL6, provided by CRL) were used for the experiment. Before the experiment, the animals were group-housed (2–5 animals/cage) with access to food and water ad libitum. Animals were maintained on a 12/12-hour light/dark cycle in a temperature- (22°C±2°C) and humidity- (approximately 50%) controlled room. Experiments were approved by the Institutional Animal Care and Use Committee of CRL.
Compounds. Fluoxetine hydrochloride and propofol were provided by CRL. Fluoxetine hydrochloride (correction factor=1.118) was dissolved in 0.9-percent saline fresh on each dosing day at a concentration of 2mg/mL and administered intraperitoneally at a volume of 10mL/kg, resulting in a daily dose of 20mg/kg. Propofol was preformulated by the supplier at 10mg/mL and injected intraperitoneally at either 3.5 or 5mL/kg, resulting in an acute dose of 35 or 50mg/kg. All solutions appeared clear upon dosing.
Experimental procedure. The mice were divided into two cohorts such that each treatment group (Table 1) was equally represented between the two cohorts. The dosing of each cohort was initiated on a staggered basis. Day 1 corresponded to the first day of pretreatment for both cohorts.
Mice received pretreatment with either saline or fluoxetine (20mg/kg, daily) intraperitoneally for 21 (Cohort 1) or 24 days (Cohort 2). Approximately 24 hours after the last pretreatment administration (i.e., Day 22 for Cohort 1 and Day 25 for Cohort 2), the animals were brought to the experimental room and allowed to acclimate for at least 60 minutes prior to the beginning the experiment. At this time, mice received intraperitoneal treatment with either saline or propofol (35mg/kg or 50mg/kg) and returned to their home cage. Forty-five minutes later, the mice were placed in cylindrical containers (24cm high×15cm diameter) filled half-way with warm water (26°C±2°C) such that animals were unable to touch or feel the bottom. After a five-minute session, the mice were removed from the water, dried with a towel, and allowed to recover on a heating pad before being returned to their home cages. The water was changed between runs.
Behavioral data analysis. The duration of animal immobility (in seconds [s]) during the FST was evaluated: a mouse was considered immobile when it ceased struggling and remained floating in the water making only those movements necessary to keep its head above water. Immobile behavior was scored using a custom video-analysis software program.
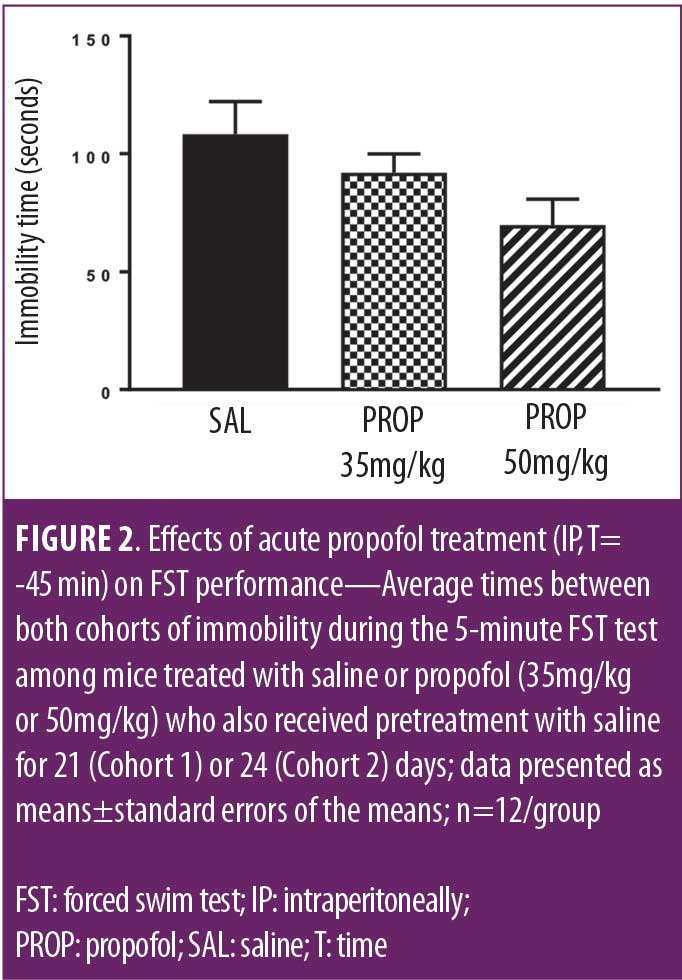
Data were represented as means±standard errors of the means using the Prism software program (Graphpad Software, La Jolla, California). Grubb’s test was used to identify statistically significant outliers.20 Outliers were excluded from data analysis and representation. Statistical analyses for Cohorts 1 and 2 were conducted separately using the SigmaStat software (Systat Software, Inc., San Jose, California). A two-way analysis of variance (ANOVA) was used to analyze the pretreatment (saline or fluoxetine) and treatment (saline or propofol) effect on immobility time. Dunnett’s post-hoc test was used for individual comparisons. In addition, results from groups saline–saline, saline–propofol 35mg/kg, and saline–propofol 50mg/kg in Cohorts 1 and 2 were combined and analyzed using a one-way ANOVA followed by Dunnett’s post-hoc test. Significance was set at p<0.05.
Results
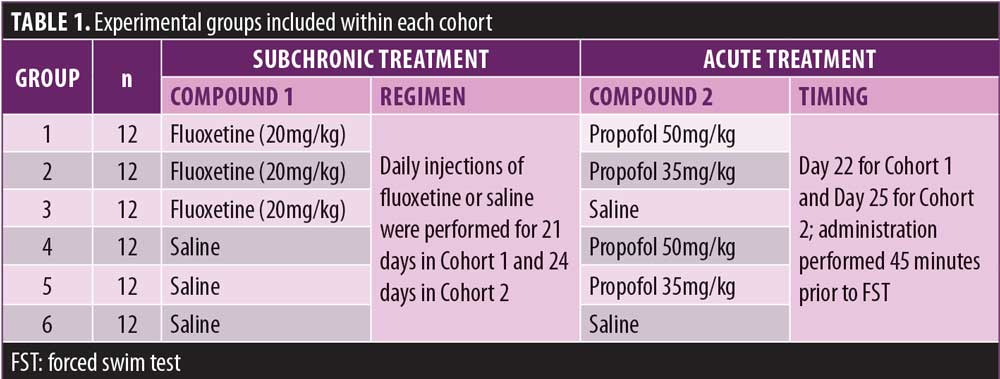
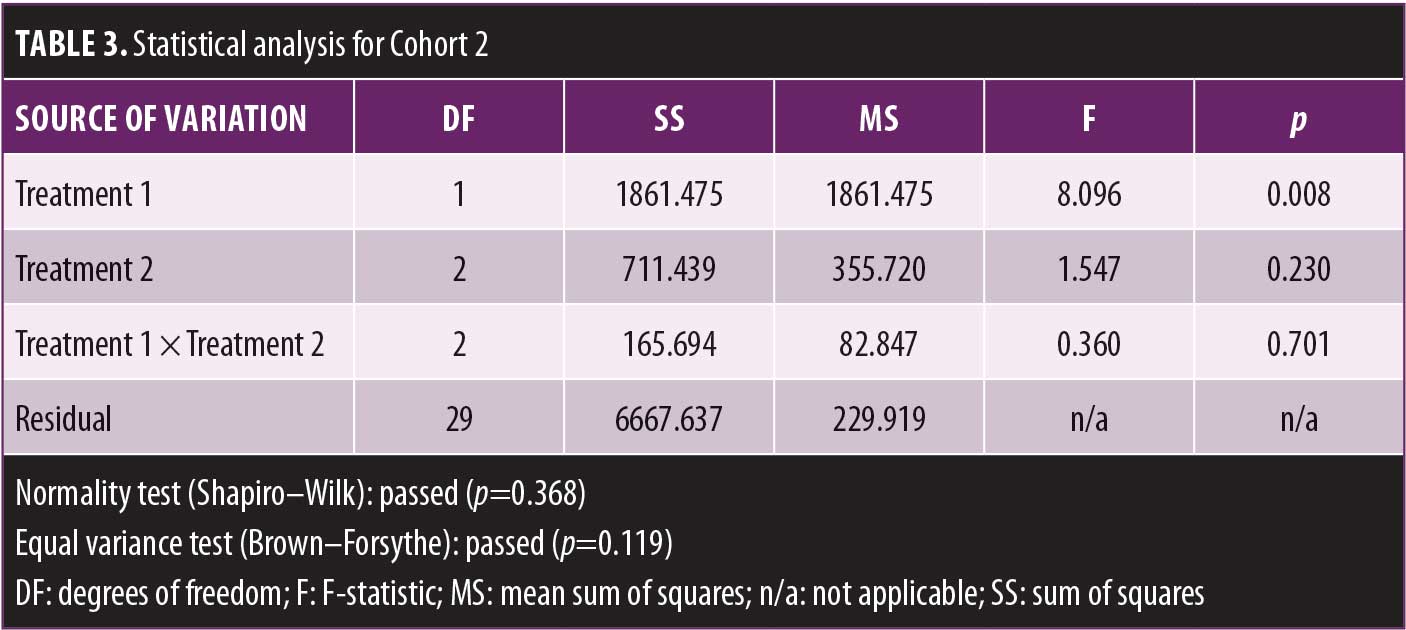
All mice successfully completed the FST. Propofol was observed to have a rapid and transient sedative effect, with mice being unresponsive for up to ~20 minutes after administration. Individual observations were not recorded. Animals were fully awake when placed in the FST containers. The effects of treatment on the time spent immobile during the FST are shown in Figure 1A for Cohort 1 and Figure 1B for Cohort 2.
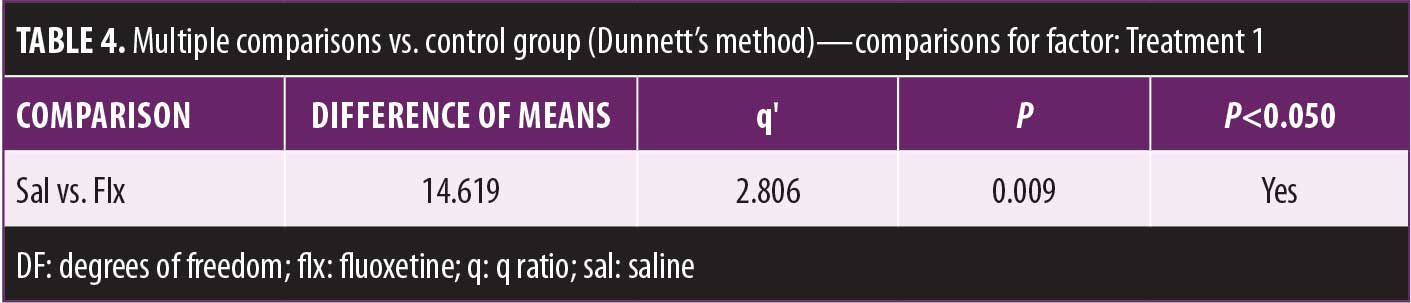
A two-way ANOVA revealed no significant effect of treatment for Cohort 1. A separate two-way ANOVA indicated that pretreatment affected immobility time independently of treatment.
Dunnett’s post-hoc comparison revealed that fluoxetine-treated mice spent more time immobile than did those in the saline groups.
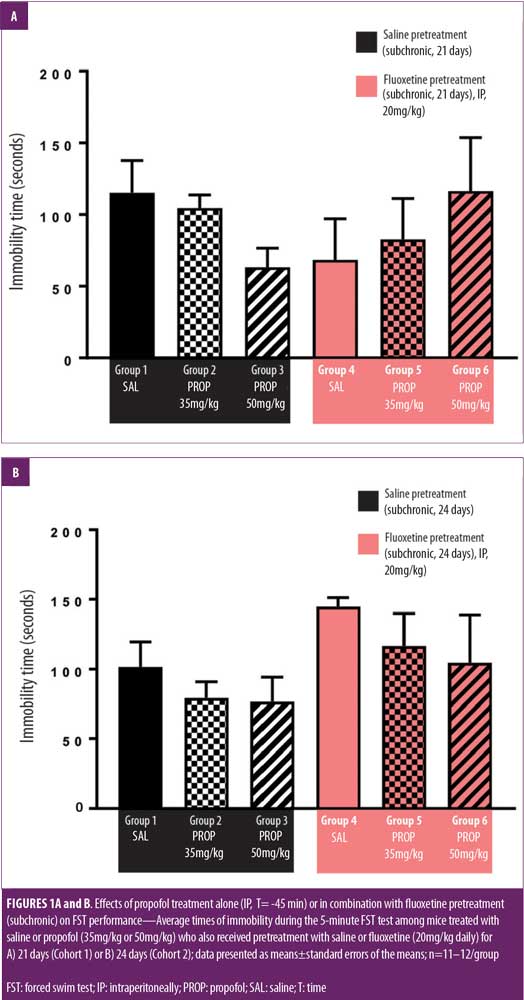
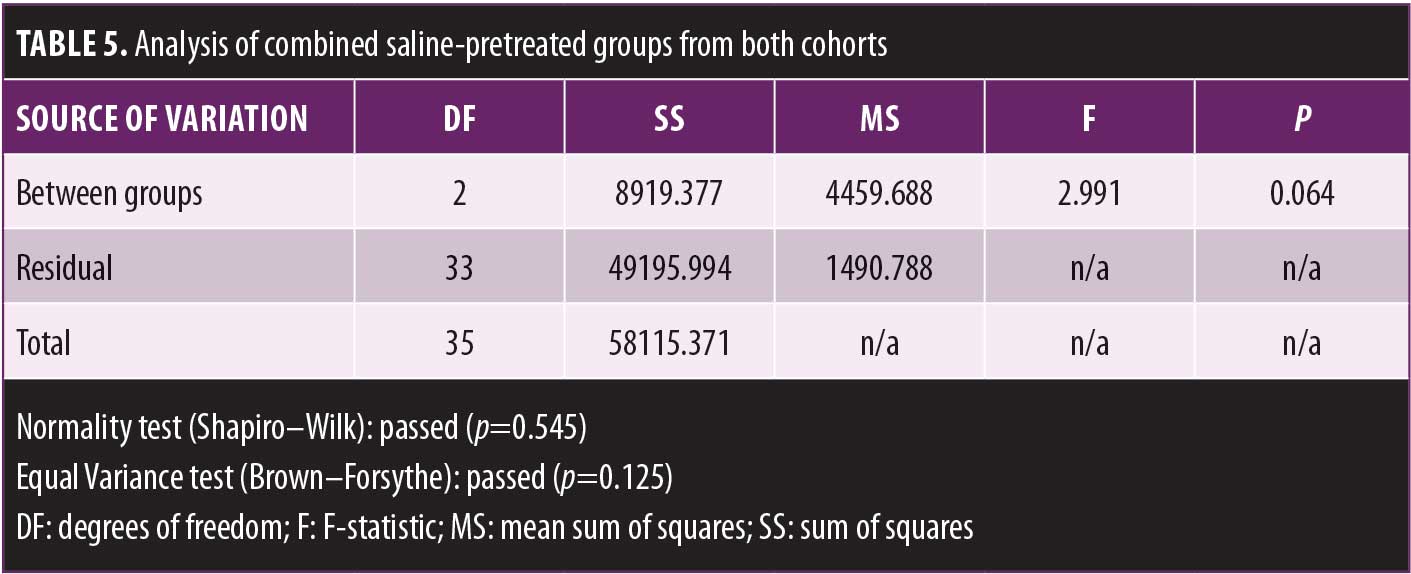
Combined results from the saline–saline, saline–propofol 35mg/kg, and saline–propofol 50mg/kg groups in Cohorts 1 and 2 are presented in Figure 2.
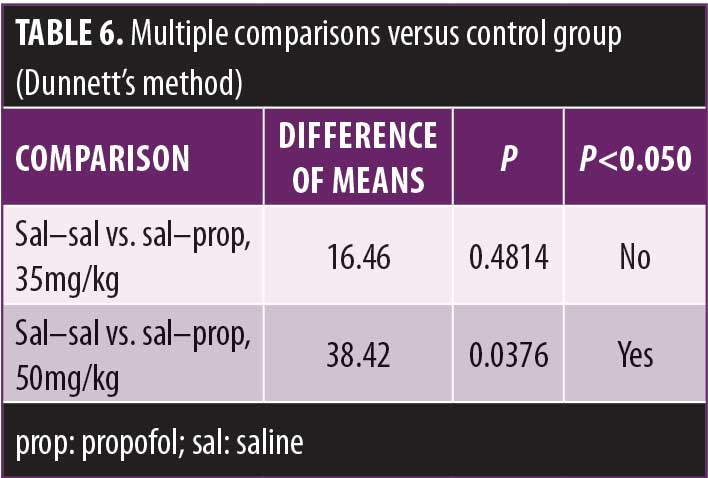
A one-way ANOVA indicated a trend toward a statistically significant effect of propofol on immobility time. Further individual comparisons using Dunnett’s method revealed that propofol 50mg/kg (p<0.05) but not 35mg/kg (p=not significant) reduced immobility time compared to the control group.
See Tables 2–6 for summaries of data analysis.
Discussion and Limitations
The present study tested the effects of acute propofol (35mg/kg or 50mg/kg, intraperitoneally) treatment alone or in combination with subchronic fluoxetine (20mg/kg, intraperitoneally, for 21 or 24 days) on FST performance in C57Bl/6 mice. Saline-pretreated mice injected with propofol at 50mg/kg displayed reduced immobility time compared to those in the saline–saline group.
The FST is a commonly used animal screening model for antidepressant effects that assesses the time an animal will swim before becoming immobile in presumed despair.16,21 However, the FST has also been conceptualized as a model of stress-coping, and the current results can be alternately viewed as consistent with propofol delaying despair or increasing emotional resilience in mice under stress.22 The implications of a single animal model to human disease are always tenuous at best and problematic to interpret, especially when unreplicated. However, the current results, if replicated, raise the question of potential clinical implications for the rapid treatment of depression and acute suicidality in humans. Appropriate additional testing would include social defeat stress models of depression, for example.
The acute intraperitoneal injection of fluoxetine 30 minutes prior to administration of the FST in C57Bl/6 mice has previously been shown to significantly improve FST performance.23 In contrast, both the subchronic and chronic (>24 days) administration of fluoxetine are more problematic to interpret. Mouse strain–specific influences in responsivity to chronic and subchronic fluoxetine treatment have previously been reported by Dulawa et al,24 who showed that chronic fluoxetine treatment failed to decrease immobility time during the FST in C57Bl/6 mice. Our findings are consistent with Dulawa et al24 in that the C57B1/6 strain of mice pretreated with fluoxetine in our experiment failed to show diminished immobility time compared to saline.
Conclusion
The results of propofol alone compared to propofol with saline in the current mouse experiment are consistent with an animal model of rapidly acting antidepressant or increased resiliency effect. Consistent with the idea that propofol might have antidepressant effects in humans are the results of a recently reported small, open-label study by Mickey et al25 of 10 patients with treatment-resistant depression, six of whom improved 50 percent or more on the 24-item Hamilton Depression Scale after receiving a series of 10 propofol infusions.25 Despite the limitations of the current preclinical experiment and Mickey et al’s modest open-label findings, additional exploratory preclinical and clinical work to investigate the potential antidepressant effects of propofol may be warranted.
References
- World Health Organization. Depression and other common mental disorders: global health estimates. Available from: https://www.who.int/mental_health/management/depression/prevalence_global_health_estimates/en/. Accessed January 14, 2019.
- Garay, RP, Zarate CA, Charpeaud T, et al. Investigational drugs in recent clinical trials for treatment-resistant depression. Expert Rev Neurother. 2017;17(6):593–609.
- Mrazek DA, Hornberger JC, Altar CA, Degitar I. A review of the clinical, economic, and societal burden of treatment-resistant depression. Psychiatr Serv. 2014;65(8):
977–987. - Gerhard DM, Duman, RS. Rapid-acting antidepressants: mechanistic insights and future directions. Curr Behav Neurosci Rep. 2018;1(5):36–47.
- Moskal JR, Burgdorf JS, Stanton PK, et al. The development of rapastinel (formerly GLYX-13); a rapid acting and long lasting antidepressant. Curr Neuropharmacol. 2017;15(1):47–56.
- Zarate CA, Machado-Vieira R. Ketamine: translating mechanistic discoveries into the next generation of glutamate modulators for mood disorders. Mol Psychiatry. 2017;22(3):324–327.
- Vidal S, Gex-Fabry M, Bancila V, et al. Efficacy and safety of a rapid intravenous injection of ketamine 0.5 mg/kg in treatment-resistant major depression: an open 4-week longitudinal study. J Clin Psychopharmacol. 2018;38(6):590–597.
- Glue P, Neehoff SM, Medlicott NJ, et al. Safety and efficacy of maintenance ketamine treatment in patients with treatment-refractory generalised anxiety and social anxiety disorders. J Psychopharmacol. 2018;32(6):663–667.
- Trapani GM, Altomare C, Sanna E, et al. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr Med Chem. 2000;7(2):
249–271. - Marik PE. Propofol: therapeutic indications and side-effects. Curr Pharm Des. 2004;10(29):3639–3649.
- Kotani Y, Shimazawa M, Yoshimura S, et al. The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther. 2008;14(2):95–106.
- Mark S, Rennie C. Propofol. A review of its pharmacodynamic and pharmacokinetic properties and use as an intravenous anaesthesia. Drugs. 1988;35(4):334–372.
- Wu Q, Zhao Y, Chen X, et al. Propofol attenuates BV2 microglia inflammation via NMDA receptor inhibition. Can J Physiol Pharmacol. 2018;96(3):241–248.
- Zhou J, Wang F, Zhang J, et al. The interplay of BDNF-TrkB with NMDA receptor in propofol-induced cognition dysfunction: mechanism for the effects of propofol on cognitive function. BMC Anesthesiol. 2018;18(1):35.
- Imperato A, Dazzi L, Obinu MC, et al. Inhibition of hippocampal acetylcholine release by benzodiazepines: antagonism by flumazenil. Eur J Pharmacol. 1993;238(1):135–137.
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl). 2005;177(3):245–255.
- Yang B, Zhang JC, Han M, et al. Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl). 2016;233(19-20):3647–3657.
- Dong C, Zhang JC, Yao W, Ren Q, et al. Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: comparison with ketamine. Int J Neuropsychopharmacol. 2016;20(3):228–236.
- Morisot N, Janssens HB. Testing the behavioral effect of propofol, fluoxetine or their combination in mice using the forced swim test (report no. 1680). San Francisco, CA: Charles River Laboratories; 2018.
- Graphpad Software Detecting outliers with Grubb’s test. Available at: https://www.graphpad.com/support/faqid/1598/. Accessed January 14, 2019.
- Yankelevitch-Yahav R, Franko M, Huly A, Doron R. The forced swim test as a model of depressive-like behavior. J Vis Exp. 2015;2(97).
- De Kloet ER, Molendijk ML. Coping with the forced swim stressor: towards understanding an adaptive mechanism. Neural Plast. 2016;2016:6503162.
- Jin ZL, Chen XF, Ran YH, et al. Mouse strain differences in SSRI sensitivity correlate with serotonin transporter binding and function. Sci Rep. 2017;7(1):8631.
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29(7):1321–1330.
- Mickey BJ, White AT, Arp AM, et al. Propofol for treatment-resistant depression: a pilot study. Int J Neuropsychopharmacol. 2018;21(12):1079–1089.





