
by Anca Maria Bejenaru, MD, and Narpinder Kaur Malhi, MD
Drs. Bejenaru and Malhi are with the Department of Psychiatry and Behavioral Health, Christiana Care in Wilmington, Delaware.
Funding: No funding was provided for this study.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Innov Clin Neurosci. 2022;19(4–6):11–22.
Abstract
Objective: Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive diagnostic and therapeutic technique that has showed benefits in various psychiatric disorders. Although there is a large body of literature available on its use in adult populations, existing research in pediatric populations is very limited. Current research has primarily focused on its use in adolescent treatment-resistant depression. However, recently, rTMS has gained attention among researchers to find its utility in other neuropsychiatric disorders, such as autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD), obsessive compulsive disorder (OCD), tics, and psychosis. There is a lack of systematic data on the safety of rTMS in children and adolescents. The aim of this article was to present an overview of the existing literature on the use of rTMS in children and adolescents and examine the relevant safety considerations.
Methods: We conducted a literature review of the English literature in PubMed on TMS in children and adolescents, using comprehensive search terms and expanding our review to include sources cited by these reports. We reviewed the application of rTMS in psychiatric disorders in the pediatric population.
Results: rTMS has been used for depression and anxiety disorders, OCD, ADHD, Tourette syndrome/tics, ASD, and schizophrenia, with variable results.
Conclusion: rTMS is a promising treatment in children and adolescents with psychiatric disorders, although larger, sham-controlled, randomized, controlled trials (RCTs) will be required to definitely demonstrate efficacy, as well as to support a safety profile.
Keywords: Transcranial magnetic stimulation, rTMS, children, adolescent, depression, autism, ADHD, tics, safety
Transcranial magnetic stimulation (TMS) is a noninvasive diagnostic and therapeutic technique that uses fluctuating extracranial magnetic fields to generate cortical currents that focally stimulate the cerebral parenchyma by depolarizing the neural cell membrane to subsequently modulate or measure the cortical excitability.1 The United States (US) Food and Drug Administration (FDA) approved TMS in 2008 for major depression and expanded the use to obsessive compulsive disorder (OCD) in 2018 and smoking cessation in 2020. Also, it has been explored for the off-label treatment of other psychiatric disorders, such as schizophrenia, posttraumatic stress disorder (PTSD), attention deficit hyperactivity disorder (ADHD), and autism spectrum disorder (ASD).
In TMS, time-varying currents are produced in an induction coil with a subsequent magnetic field that generates a secondary electric current with direct depolarization of neural structures, subsequently evoking action potentials (single stimuli) or modulation of the cortical excitability.2
There are three types of TMS protocols that have been used, each with different specifications. Single-pulse (spTMS) and paired-pulse TMS (ppTMS) protocols measure motor cortex excitability and have been utilized in the adolescent population to get insight on the neurophysiology of certain disorders. For example, spTMS has been used in psychiatry for the research of transcallosal inhibition in children with ADHD and evidenced the cerebral response to psychopharmacologic treatment.3 ppTMS has been applied in the evaluation of the intracortical gamma-aminobutyric acid type A (GABAA) receptor-mediated inhibition and excitatory glutamatergic neurotransmission. ppTMS has underlined the pathophysiology of ADHD in studies of children4,5 and has showed differences among ASD subtypes.6,7
Unlike spTMS and ppTMs, repetitive TMS (rTMS) is used to measure cortical plasticity and has therapeutic implications by applying repeated pulses at an established frequency or discharges of stimulation. rTMS has longer lasting effects on cortical excitability and, subsequently, clinically, for weeks to months. This is related to the frequency and number of rTMS administrations, as well as site and intensity of stimulation.8 If the time between pulses is one second or more (1Hz or low-frequency [LF]-rTMS), rTMS has an inhibitory cortical effect, whereas if the frequency is equal or greater than 5Hz (high-frequency [HF]-rTMS), it produces an excitatory result.9
This electrical stimulation can produce long-term depression (LTD) or long-term potentiation (LTP) at the level of synaptic transmission, and it not only modulates the activity at the site of stimulated area, but impacts circuits that propagate to distant structures as well.10
Studies have suggested that neurotransmitter systems are implicated in the clinical response to rTMS, especially in depression. HF-rTMS evidenced serotoninergic modifications, with a positive relationship of 5-hydroxytryptamine 2A (5-HT2A) receptor binding levels in the bilateral dorsolateral prefrontal cortex (DLPFC) and a negative relationship with right hippocampal 5-HT2A receptor uptake measures.11 Regarding dopamine, it was shown that prefrontal rTMS can generate dopamine release in the mesostriatal, mesolimbic, and striatal regions,12 while GABAergic metabolism in the brain may be influenced by rTMS, with DLPFC independently expressing the metabolic changes.13 Following just a single HF-rTMS session, Baeken et al14 showed clinical improvement that was connected to significant GABA concentration raise in the left DLPFC (L-DLPFC).
TMS was initially used in children in the early 2000s with diagnostic and therapeutic targets. Therapeutic applications of TMS have been used in children with depression and anxiety, ADHD, Tourette syndrome (TS), ASD, and schizophrenia, as well as neurological disorders, such as epilepsy, cerebral palsy, and stroke. Despite promising evidence of rTMS in adults, with accelerated TMS trials,15 the majority of rTMS research on children and adolescents is limited to case reports and open-label studies. Safety, ethical concerns, and a thoughtful approach are paramount in such studies, given neurodevelopmental concerns.16,17
Despite promising outcomes and availability of literature supporting the use of rTMS in adults, it has very limited use in children and adolescents.16,18 The purpose of this article was to gather available evidence to provide an overview of the use of rTMS in child and adolescent psychiatry.
Methods
A literature search was conducted in PubMed (inception–December 31, 2020) to review the literature. Search terms included “rTMS,” “transcranial magnetic stimulation,” “child and adolescent,” “depression,” “mood disorders,” “anxiety disorders,” “attention deficit hyperactivity disorder,” “autism spectrum disorder,” “schizophrenia,” “Tourette disorder,” and “tics.” Articles that discussed the use of TMS in patients under the age of 21 years were included, in addition to articles discussing the safety of TMS in the same population. We identified an initial 110 articles, reviewing the abstracts, titles, or full text and removing the articles that did not meet the criteria. In addition, we also searched reference lists of the included studies and relevant review articles.
Safety of rTMS
Significant aspects of TMS include the possible adverse side effects of the treatment, with most of the guidelines being focused on adults, although its use in children and adolescents has been studied as well.
Seizure is the most severe concern of TMS, although it has a low incidence of occurring and usually affects patients with predisposing factors, such as previous neurological disorders, including epilepsy and stroke. Taking into account the possible side effects, Hong et al19 compared spTMS and ppTMS with theta burst stimulation (TBS), respectively, in 165 participants between the ages of 6 to 18 years (of participants receiving TBS, 68% were healthy controls, 25% had a diagnosis of TS, and 7% had other motor disorders; of participants receiving sp/ppTMS, 21% were healthy controls and 79% had ADHD), and some participants were actively taking neuropsychiatric medications. There were no reported seizures or serious adverse events in all who completed sp/ppTMS or TBS sessions. The reported adverse events occurred in 10.5 percent of participants in the TBS group, compared to 12.4 percent in the sp/ppTMS group, and were rated as mild or minimal.19
Prior reviews of existing literature regarding the safety of TMS in children and adolescents showed that side effects are typically mild, with temporary headaches, skull discomfort, twitching, mood changes, fatigue, and tinnitus, similar to those noticed in adults.20
Per our review, observed adverse reactions were scalp pain/discomfort;21–29 headache;22,24,26–35 dizziness;22,33 musculoskeletal or neck discomfort, pain or stiffness, or chest tightness;22,29–31 nausea, gastrointestinal symptoms, or nasopharyngitis;22,29 eye or jaw twitching;22,32 fatigue or restlessness;24,35 new onset self-remitted hypomanic episodes;34,36 seizures (one case in the context of alcohol use);25,36,37 and suicidal ideation.38
A different type of adverse event occurred in the case of a 15-year-old female patient diagnosed with Charles Bonnet syndrome after application of six sessions of LF-rTMS at the right DLPFC (R-DLPFC) for depression (not treated pharmacologically). The patient presented with intense, intricate, and colorful visual hallucinations with preserved insight that remitted about a week after cessation of rTMS without pharmacological intervention.39
The main concerns with using TMS in children or adolescent populations are the unknown consequences of such stimulation in the still evolving cerebrum and the possible impact on neurodevelopment in the context of existing limited data, as well as the ethical dispute when working with this age group. Although sp/ppTMS options are considered relatively safe, experts have raised concerns about distinctive effects of TMS on neurodevelopment.40 rTMS international consensus panels have concluded that sp/ppTMS research is safe for children two years of age and older and that children should not participate as subjects in rTMS protocols without compelling grounds, such as the treatment of refractory psychiatric or neurological conditions.41
Mood and Anxiety Disorders
As per the National Institute of Mental Health (NIMH), the prevalence of major depressive episodes among adolescents in 2017 was found to be 13.3 percent of the US population, and adolescents between the ages of 12 to 17 years were identified as having had at least one major depressive episode, with a higher prevalence in female adolescents (20.0%) compared to male adolescents (6.8%). This rate was highest in adolescents who reported two or more races (16.9%).42 Of the adolescents who had experienced at least one major depressive episode, 70.77 percent, or 9.4 percent of the US population, presented with major depressive disorder (MDD) causing severe impairment. Approximately 19.6 percent were treated by a healthcare professional only, while another 17.9 percent received combined care with added pharmacological treatment. A total of 2.4 percent were treated with medications, while 60.1 percent of adolescents who had experienced major depressive episode had no treatment.42
Evidence-based treatment of depression is imperative to prevent the development of treatment-resistant depression. Despite advances in treatment for adolescent depression, such as medication and psychotherapy, it is estimated that 30 to 40 percent of adolescents with depression do not show adequate clinical response, which is defined as at least a 50 percent reduction in depression, and have a high likelihood of recurrence in adulthood.43,44
Limitations in pharmacological treatment in children and adolescents with MDD and other psychiatric disorders are imposed by the FDA’s warning for increased risk of suicidal thinking and behavior with antidepressants.45
Multiple reviews have demonstrated the statistically significant efficacy and safety of rTMS in adult depression, but when considering the younger age bracket, clear recommendations are still in the development phase. Table 1 summarizes all case reports and open-label studies for adolescents with depression.
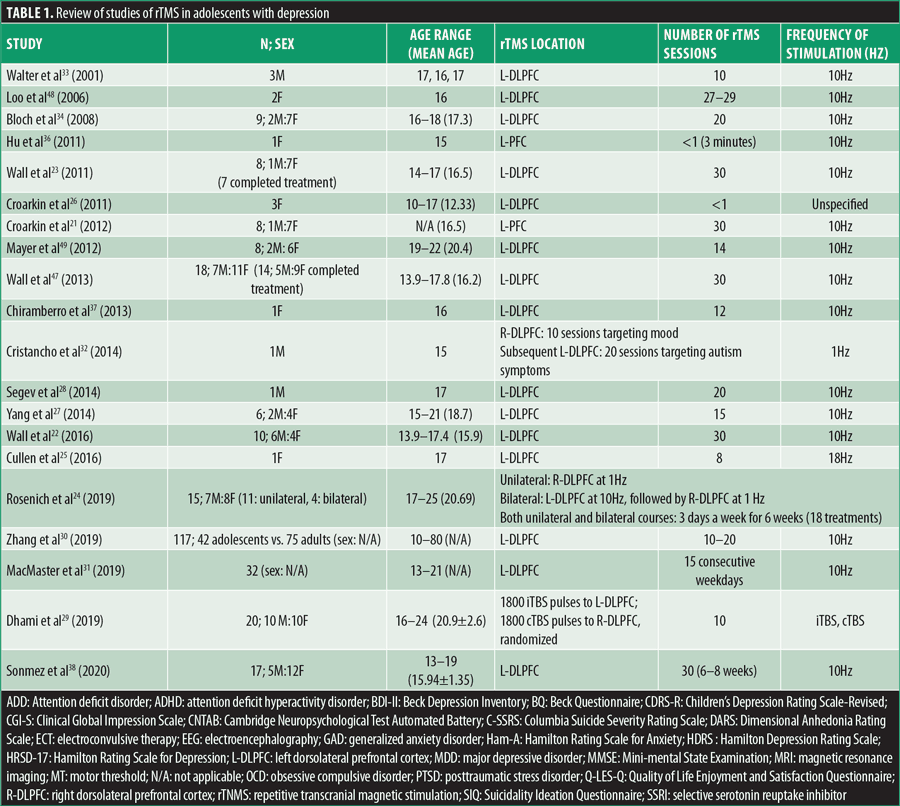
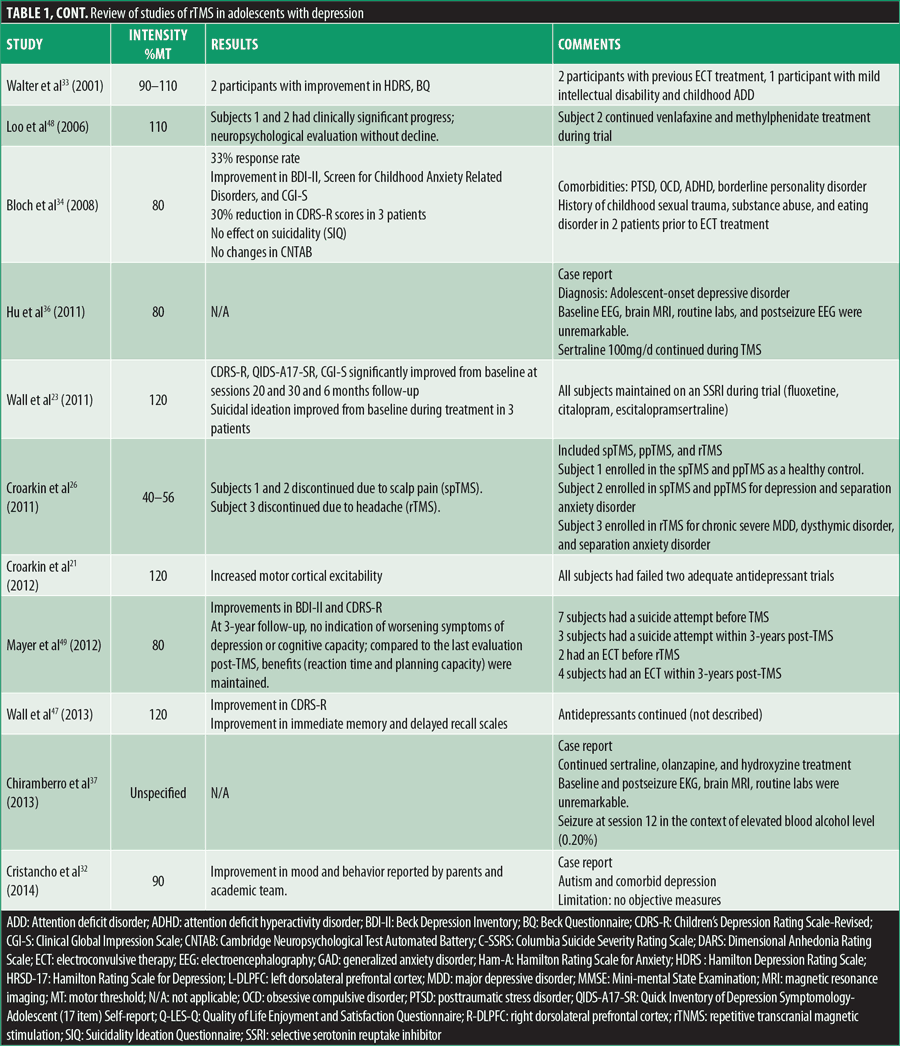
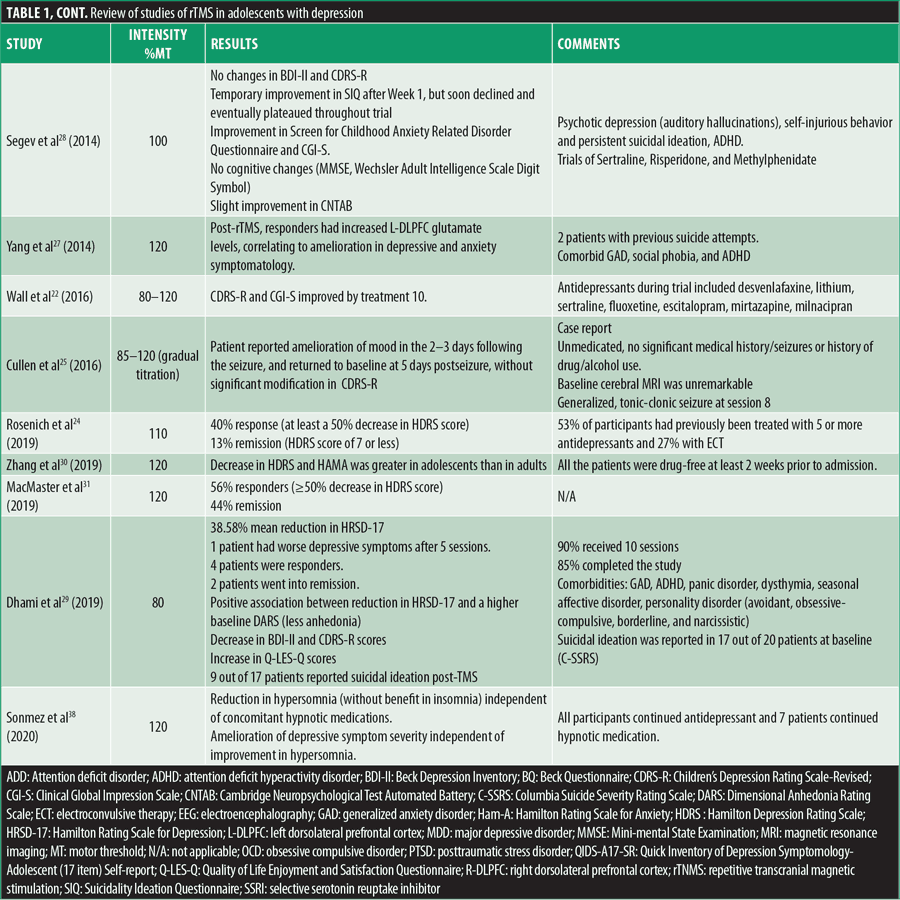
Cortical excitability can be increased by HF-rTMS longitudinally (decreasing the motor threshold) as per a study by Croarkin et al,21 in which eight adolescents with treatment-resistant MDD were treated with add on prefrontal HF-rTMS, where cortical excitability was measured with resting motor thresholds. The same author, presuming that adolescents with depression have an underlying glutamate-glutamine cycle disturbance, evaluated the glutamine/glutamate ratios at baseline, posttreatment, and six-months follow-up in adolescents with treatment-refractory MDD who were treated with HF-rTMS (30 sessions).46 Proton magnetic resonance spectroscopy scans of the anterior cingulate cortex and L-DLPFC evidenced that glutamine/glutamate ratios rose in concordance with melioration of depressive symptoms after treatment and six-month follow-up while reaching statistical significance in L-DLPFC.46 Glutamate levels in L-DLPFC were furthermore measured with short echo proton magnetic resonance spectroscopy before and post-rTMS in a different trial, while assessing changes in depression and anxiety symptomatology.27 Four out of the six participants in this study were treatment responders, resulting in mean reductions of 68 percent in Hamilton Depression Rating Scale (HDRS) scores and 84 percent in Beck Depression Inventory (BDI-II) scores, with one participant being a responder to HDRS only. Regarding anxiety, responders presented with a mean decrease of 78 percent in Hamilton Rating Scale for Anxiety (Ham-A) scores, while smaller redcutions were seen in nonresponders. Responders to the HDRS and BDI-II had lower baseline glutamate measures, while after treatment an increase of 11 percent in glutamate was evidenced. Nonresponders had more elevated baseline glutamate levels that eventually decreased by 10 percent. Post-rTMS, L-DLPFC glutamate concentrations increased, correlating with the amelioration in depression and anxiety in responders.27 Meaningful response/remission rates in symptomatology were observed to be more prominent in adolescents compared to adults, when analyzing 42 adolescents compared to 75 adults with mood or anxiety disorders, where symptoms were measured with the HDRS and Ham-A at baseline after two and four weeks of follow-up after treatment with rTMS.30
In teenagers, it is known that one of the leading causes of death is suicide (11%),47 and certain trials assessed suicidality and its correlation with rTMS. This topic was evaluated in one study at baseline and after 10, 20, and 30 treatments of HF-rTMS with the Columbia Suicide Severity Rating Scale (C-SSRS) Intensity of Ideation subscale and Item 13: Suicidality on the Children’s Depression Rating Scale-Revised (CDRS-R). The predicted odds of suicidal ideation were reduced over six weeks of treatment, but this was considered to be not meaningful when adapted for depression severity.48 An older study also evidenced that TMS had no significant impact on suicidality, while the anxiety measurement (Screen for Child Anxiety-Related Disorders questionnaire) was dependent on rTMS therapy stage with decreased measurements of anxiety only at the end point of therapy and at one-month follow-up, compared to baseline;23,34 the study described eight patients, of which only seven completed the treatment (and which included participants nonresponsive to 2 adequate antidepressant trials), with resultant significant betterment in CDRS-R from baseline to session 10 and continued improvement at sessions 20 and 30 and six-month follow-up. Expression of suicidal ideation declined as treatment advanced, with only one patient reporting passive death wish and, at six-month follow-up, self-cutting behavior (in the context of failed relationship). One participant was hospitalized five-weeks post-TMS, unrelated to TMS treatment (death of best friend).
Contrary to the results of most studies, Segev et al28 described an adolescent with psychotic depression (auditory hallucinations), self-injurious behavior, and persistent suicidal ideation since childhood, with a pharmacological regimen consisting of sertraline, risperidone, and methylphenidate for his ADHD. He was treated with rTMS and had three follow-up visits, one-, two-, and four-weeks posttreatment completion. As a result, BDI-II and CDRS-R scores did not evidence a statistically meaningful difference from baseline to the end of follow-up. The Suicidality Ideation Questionnaire showed temporary improvement after Week 1 but declined and eventually plateaued post-Week 3. He did present with betterment in anxiety throughout treatment (Screen for Childhood Anxiety Related Disorder Questionnaire) and in Clinical Global Impression (CGI). Cognitively, he was stagnant (Mini-mental State Examination [MMSE], Wechsler Adult Intelligence Scale Digit Symbol, or Cambridge Neuropsychological Test Automated Battery [CNTAB]), with slight progress in CNTAB. As opposed to the objective results, the patient’s psychiatrist and therapist considered that he actually improved significantly, as the patient was less focused on his suicidal ideations.28
An important topic when considering rTMS in this population is the possible advantages or negative consequences on neurocognitive functioning. Per a study by Wall et al49 consisting of 18 patients with MDD with a mean age of 16.2 years, 14 completed a 30-session rTMS course over 6 to 8 weeks, with subsequent improvement in depression; they also showed statistically significant betterment in immediate memory and delayed recall, as assessed by the Children’s Auditory Verbal Learning Test (CAVLT), while interference, level of learning, or immediate recall remained steady, without meaningful modifications in the Delis-Kaplan Executive Function System (D-KEFS) Trail Making Test (TMT).49 Additionally, Bloch et al34 evaluated cognitive function in adolescents with CNTAB, and planning improved one month after completion of rTMS, while reaction time was enhanced immediately after cessation and continued at one month.
In the pediatric study by Loo et al,50 Subject 1 received 29 rTMS sessions over six weeks, while Subject 2 missed one session per week, with a 13-day interference in the trial at the end of the Week 4 blind phase, but continued with another 20 sessions in Week 5. Both subjects showed clinically significant improvements that were maintained months post-TMS, but especially noteworthy is the progress during the blind four-week phase. Neuropsychological evaluation at baseline, post-20 treatments, and at the end of trial (Wechsler digit span forwards and backwards and digit symbol modalities test, Rey Auditory Verbal Learning Test, Controlled Oral Word Association Test, TMT A and B) was without decline in the functioning areas (memory, attention and concentration, frontal executive function, and psychomotor speed).
A distinct neurocognitive evaluation at baseline, completion of TMS, and six-month follow-up completed in six out of seven patients did not show statistically relevant decline in immediate memory, level of learning, immediate recall, delayed recall, or auditory/verbal fluency. Autobiographical Memory Interview outcomes (a tool used to investigate retrograde amnesia by investigating the nature and extent of the memory deficit) demonstrated a reduction in scores at treatment completion and at six-month follow-up in two subjects.23
Long-term effects of rTMS on treatment-resistant depression and cognitive functioning were assessed by Mayer et al,51 and at three-year follow-up, there was no indication of deterioration in symptoms of depression or cognitive capacity. Compared to the last evaluation post-TMS, the benefits were maintained (reaction time and planning capacity). Three out of eight participants ameliorated with the greatest change by one-month posttreatment; another four patients had a waxing and waning progress pattern, and one patient presented with chronic dysfunctional course.51
When looking for alternatives, more recent research has developed theta burst stimulation (TBS) dosing that would decrease the time burden of treatment, compared to the time consuming and possible adverse events caused by a higher intensity of stimulation with regular rTMS; TBS functions by delivering bursts of three high frequency pulses (50Hz), with pauses between stimuli of 200ms (5Hz) at 80 percent active motor threshold, and can be in a continuous pattern without pauses (cTBS) for a total of 20 to 40 seconds for 300 to 600 pulses, or intermittent pattern (iTBS) every eight seconds for total of three minutes for 600 pulses, versus 37.5 minutes for a standard 10Hz session. cTBS is believed to reduce cortical excitability, while iTBS is believed to increment it. iTBS is commensurate to standard 10Hz rTMS in terms of being efficient, safe, and tolerable.52
TBS is comparable to the endogenous cerebral theta rhythms and could affect cerebral plasticity by producing long-term potentiation and depression-like activity,53 with more lasting effects than regular rTMS. Another application of TBS could be bilateral treatment without much prolongation in the duration of the session.54 Combined cTBS and iTBS could lead to higher antidepressant results, compared to sham or unilateral applications in adults with treatment refractory depression.55
An elevated resting-state functional connection amongst prefrontal and striatal areas is the characteristic of OCD, with TMS trials usually targeting the R-DLPFC, supplementary motor area, or orbital frontal cortex. In adolescent populations, there is a paucity of such studies. One such study examined the rTMS application in 18 patients aged 12 to 18 years with OCD, using active and sham groups and comparing functional MRI changes post-1Hz rTMS (1,800 pulses, 110% of resting motor threshold [RMT]) in a single session lasting 30 minutes, applied over the R-DLPFC, while inducing OCD-related anxiety. The study did not find distinctions in neural activity poststimulation compared to prestimulation.56
Schizophrenia
Several studies evidenced LF-rTMS targeting the left temporoparietal cortex (LTPC) as being effective in decreasing auditory hallucinations in schizophrenia,57 yet regarding other psychotic and negative symptoms or cognitive function, results have been less conclusive.58,59 Other studies have used the HF-rTMS localized at the L-DLPFC to address negative symptoms,57,60,61 again with various results with nonmeaningful effect size on positive or negative symptoms. Nonetheless, one of these studies showed that participants with more pronounced negative symptomatology at baseline would respond better to rTMS and that rTMS had an inferior effectiveness on negative symptoms in a lengthier illness.62
In the pediatric population with schizophrenia, the benefits of rTMS have been brought to attention mostly by descriptions of multiple case reports. Jardri et al63 described the cessation of auditory hallucinations in an 11-year-old male patient diagnosed with very early-onset schizophrenia, without any previous sign of pervasive developmental disorder, who had a prior functional MRI scan that showed bilateral neural activity in the auditory cortex during auditory verbal hallucinations. He successfully received functional MRI (fMRI)-guided LF-rTMS over the LTPC but required repeat regular sessions to maintain the effect.63
Another case report presented an 18-year-old female patient with symptoms of hopelessness, severe anxiety, and dysphoric commanding hallucinatory voice, who received LF-rTMS (1Hz) over the LTPC, with repeated protocol. The patient showed gradual improvement in auditory hallucinations, which eventually resolved completely, with subsequent improvement in mood symptoms.64 A similar case report presented an 18-year-old female patient with childhood-onset, psychopharmacological treatment-resistant schizophrenia beginning at the age of nine years. She was treated with 1Hz stimulation rTMS on the LTPC, while taking clozapine 400mg/day. She presented with a return of auditory hallucinations that increased in intensity and frequency after five-months post-rTMS, but they lessened progressively, with almost complete resolution, when rTMS was reintroduced.65
In an open-trial study of three 18-year-old male patients with schizophrenia treated with rTMS at the right frontal cortex, two patients showed improvement in Schedule for the Assessment of Negative Symptoms (SANS) and Schedule for the Assessment of Positive Symptoms (SAPS) scores, while the third patient had subjective reports of benefit in hallucinations and agitation, by the patient and parents.33
Taking in consideration the hypothesis of cerebellar dysfunction being implicated in the pathogenesis of schizophrenia (cognitive dysmetria theory),66 one open-label, uncontrolled study from 2015 described 11 subjects with recent-onset schizophrenia, with a mean age 24 years, who were treated with HF-rTMS of the cerebellar vermis. They denoted significant reduction on the Positive and Negative Syndrome Scale and depressive symptomatology as per the Calgary Depression Scale for Schizophrenia.67
Autism Spectrum Disorder (ASD)
ASD is one of the neurodevelopmental disorders with limited therapeutic options, especially when targeting its core symptoms. Researchers have been becoming more interested in TMS as a treatment method for ASD, as it was shown to modulate plasticity, one of the thought etiologies involved in ASD.68
In Oberman et al,35 the safety of TMS (TBS and single-pulse stimulation) was observed in 19 high-functioning male patients with ASD (9–18 years old), as there were no reported severe adverse events, except headache and fatigue. This study also demonstrated a positive linear correlation between age and length of response, with older age associated with prolonged effect of TBS, and with one-third of participants having a facilitatory reaction to typically suppressive cTBS, which is suggestive of a GABAergic impairment in ASD.35
Enticott et al7 investigated motor cortical inhibition and excitability in 11 patients with high-functioning autism (HFA), 14 patients with Asperger disorder (per Diagnostic and Statistical Manual of Mental Disorders, 4th edition [DSM IV] criteria), and 11 neurotypical patients by applying ppTMS, which showed significant decreased cortical inhibition in the patients with HFA, compared to patients with Asperger disorder and neurotypical patients, respectively, underlining the dysfunction of GABAA receptors. A case-control study used iTBS on motor cortex plasticity (M1) in adolescents with ASD and matched controls, provoking a relevant reduction in amplitude of excitability in the ASD group, supporting the hypothesis that patients with ASD have increased cortical excitability.68 Similarly, Baruth et al69 applied 1Hz rTMS treatments to the L-DLPFC, and subsequently to the R-DLPFC, with notable amelioration in discriminatory gamma activity between relevant and irrelevant visual stimuli in patients with ASD, reinforcing the idea that TMS has an elevated cortical inhibitory effect. Participants also showed decreased repetitive and restricted behavior pattern (Repetitive Behavior Scale) and decreased irritability (Aberrant Behavior Check), without notable modifications in social awareness or hyperactivity.69 Similar findings (except changes in irritability) were found in a different study applying 0.5Hz rTMS to the L-DLPFC in high-functioning ASD,70 as well as in Gómez et al,71 which demonstrated improvements in scores in the Autism Behavior Checklist (ABC), Autism Treatment Evaluation Checklist (ATEC), and Autism Diagnostic Interview (ADI-R). The results were maintained during the first six months posttreatment.70
In a case report of a 15-year-old male patient with autism and comorbid depression, where LF-TMS (1Hz) was applied over the R-DLPFC to address depression and over the L-DLPFC for core features of autism, amelioration in social interactivity and communication was seen, with improved mood, eye contact, and focus and increased verbal output.32 Casanova et al72 demonstrated a relevant decrease in repetitive and restricted behaviors, as well as irritability, and at the same time showed relevant changes in selective attention and executive function (total errors rate).
The Kanizsa illusory figure test was completed by participants in a trial wherein pre- and post-LF-rTMS (1Hz) was applied over the L-DLPFC for the first six treatments, with the subsequent six treatments applied over the R-DLPFC. The patients demonstrated improvement in behavioral performance, visual attention, and post-error slowing of reaction time (normalized).73 A different study from the same group also reported lower irritability and hyperactivity on the Aberrant Behavior Checklist and reduced stereotypic behaviors on the Repetitive Behavior Scale, with improved executive functioning.74
Prefrontal rTMS and electroencephalographic (EEG) neurofeedback (NFB) applied to 42 children with ASD (mean age 14.5 years) reinforced previous findings of decreased repetitive and stereotypic conduct and hyperactivity and lethargy, with improved motor response accuracy, reaction time slowing, and decreased errors.75
An open-label study that used intermittent TBS applied at the R-DLPFC in 10 right-handed male patients with ASD between the ages of 9 and 17 years showed betterment in scores of the Repetitive Behavior Scale Revised and Yale-Brown Obsessive Compulsive Scale, as well as in persistent errors on the Wisconsin Card Sorting Test and sum time for the Stroop test, along with improved compulsions and restricted and repetitive behaviors.76
Panerai et al77 presented a report from four preliminary studies on children with ASD and severe intellectual disability, with a focal point on eye-hand coordination, that covered a four-year period (each study covering 1 year). The conclusion reflected a notable increase in eye-hand performances, with HF-rTMS only applied to left premotor cortex, that were maintained for one hour posttreatment, and a superior outcome was obtained when combining HF-rTMS and eye-hand integration education, especially for memory consolidation.
A recent pilot, randomized, double-blind, parallel, controlled trial studying HF-rTMS (20Hz, randomly stimulating the L- or R-DLPFC, with immediate succeeding application to the contralateral side) in 16- to 35-year-old participants with ASD without intellectual disability (intelligence quotient ≥70, per Wechsler Adult Intelligence Scale 4th Edition [WAIS-IV]), but with significant executive function dysfunction (t-score>65 on any subscale of the Behavioral Rating Inventory for Executive Function-Self Report [BRIEF-SR] or BRIEF-Adult), did not show a relevant difference on executive function presentation (CANTAB spatial working memory total errors and Behavioral Rating Inventory for Executive Function Metacognition Index) when comparing active and sham rTMS, respectively. However, the study emphasized possible improvement in patients with more severe adaptive functioning impairment.78
Tourette syndrome/Tics
The cortical-striatal-thalamic-cortical loops are hypothesized to be involved in TS, with the supplemental motor area (SMA), which can be overactive in people with TS, having significant connections with these regions and being easily accessible to rTMS.
A meta-analysis79 that included eight studies in children and adults evaluating the effects of rTMS in tics demonstrated that rTMS significantly ameliorated tic and comorbid obsessive-compulsive symptoms in participants with TS, when compared to baseline, but not when controlled for placebo. Administration at the bilateral SMA was noted to be more effective than wider SMA, left motor, prefrontal, and bilateral premotor areas, and a younger age was correlated with a superior outcome in tic symptoms. Symptoms of ADHD were without meaningful improvement.79
Kwon et al’s80 open-label, 12-week cohort study in Asia used rTMS at 1Hz, 100 percent MT, and 1,200 stimuli per day over the SMA in 10 male children between 9 and 14 years of age with comorbid ADHD, MDD, or OCD, without a sham control group. Pharmacological treatment included escitalopram, risperidone and clonazepam. rTMS was found to be safe in all participants, except one, who presented with minimal scalp pain that resolved after one day. The result was clinically relevant, with incremental improvement in tic symptoms maintained at three-month follow-up (total Yale Global Tourette’s Syndrome Severity Scale [YGTSS] score, as well as CGI, reduced from baseline to Day 10). No meaningful modifications were noted in ADHD, depression, or anxiety.80
Le et al81 showed that r-TMS applied at 1Hz and 110 percent RMT at the SMA in 25 children aged 7 to 16 years had benefits to TS, with an important reduction in tics, ADHD, anxiety and depression symptoms (decreased YGTSS; CGI; Swanson, Nolan and Pelham Rating Scale, version IV for ADHD [SNAP-IV]; and Attention Test-error rate scores during rTMS, which stabilized at follow-up). Pharmacological treatment was maintained (topiramate, haloperidol, inosine, tiapride, trihexyphenidyl, or traditional Chinese medicine jingling liquid). Improvements were continued up to six months posttreatment in 68 percent of participants.81
A recent open-label, Phase I clinical trial82 regarding the application of 1Hz neuronavigated robotic rTMS, 100 percent RMT, over the bilateral SMA for 15 sessions in 10 patients (eight male, two female) aged 9 to 15 years old with TS resulted in improvement in YGTSS scores (impairment, motor and phonic tic severity, and total tic severity).802
Two case reports83 reporting an adult and an adolescent (16 years old) with severe TS receiving 1Hz rTMS at 110 percent RMT applied at the SMA demonstrated benefits in both participants in tics, as well as depression and anxiety, but not as much in OCD symptoms. No adverse events were noted. The adolescent patient had struggled with TS since the age of six years (motor and phonic tics, dystonia, and self-injurious behavior), as well as OCD, ADHD, and MDD, and experienced failed neuroleptics, A2-agonists, selective serotonin reuptake inhibitors (SSRIs), anticonvulsants, benzodiazepines, and nicotine patch. Fluphenazine, fluoxetine, and benztropine treatments were maintained during rTMS. Subsequent to rTMS, he presented 68 percent amelioration in YGTSS scores, and the benefit was persistent at four-month follow-up.83
Two other case reports84 described 15- and 18-year-old patients with TS. The 15-year-old female patient had no psychiatric comorbidities, had a familial history of tics in her sister and father, and failed haloperidol, risperidone, and clonidine treatments. At the time of her treatment with LF-rTMS she was on pimozide and trihexyphenidyl, and she did not present with subsequent significant amelioration in her symptoms (as per YGTSS scores), only presenting a 10 percent improvement. The 18-year-old male patient had comorbid OCD and failed trials of pimozide, risperidone, fluoxetine, clomipramine. He was on fluphenazine, trihexyphenidyl, sertraline, and clonazepam at the initiation of LF-rTMS, experiencing a resultant decrease in YGTSS and Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores, with significant benefit persistence at three-month follow-up.84
Continuous TBS was documented in a randomized, double-blind, sham-controlled trial, at 30Hz, 90 percent RMT, administered at the SMA in 12 patients aged 10 to 22 years, with TS comorbid with ADHD and OCD, while using fMRI navigation.85 cTBS was applied at eight sessions over two days, with subsequent meaningful inhibition in the motor network (SMA, bilateral M1), but without significant distinction in decreasing clinical tics.85
Attention Deficit Hyperactivity Disorder (ADHD)
TMS use in ADHD has been especially emphasized in adults, showing improvement in hyperactivity but not attention.86,87 Bloch et al88 demonstrated benefits in attention, without differences in mood, anxiety, or hyperactivity.
When considering the role of dopamine and ADHD pathophysiology, previous studies have shown that rTMS induces striatal dopamine increase, similar to use of d-amphetamine,89 and when rTMS is applied at mid-DLPFC, it leads to a release of dopamine in the ipsilateral caudate nucleus.90
Children with ADHD were reported to have reduced intracortical inhibition with normal intracortical facilitation at baseline, compared to healthy controls, which improves with methylphenidate administration.91
In the pediatric population, TMS studies have been more limited. In a mainstay study, Weaver et al92 used rTMS versus sham, applied at the right prefrontal cortex at 10Hz with a crossover stage in participants aged 14 to 21 years old, with prior medication washout. No differences were noted between sham or active TMS, but the participants that received active treatment in Phase 2 started to indicate improvement, compared to the sham group. The study showed benefits in CGI and ADHD-IV in combined active and sham TMS.92
One open trial that included younger children, aged 7 to 12 years old, receiving TMS of 1Hz at the L-DLPFC, showed statistically meaningful amelioration in behavior, such as inattentiveness in an academic context and hyperactivity/impulsivity at home. It underlined the safety of TMS in this population, with only mild adverse events being observed.93 One study with a main focus on modifications on serum microRNA (miRNA) let-7 in children aged 8.83±2.53 years old, treated either with rTMS (applied at R-DLPFC, 10Hz, 100% MT) or atomoxetine, compared to healthy controls, showed benefits in attention deficit, hyperactivity impulse, and oppositional defiance for both treatment groups.94
Limitations
As is the case with most of these studies, our article is subject to limitations. Potential limitations include a difficulty to generalize our findings to a larger population, as we have included case reports, open-label trials, and studies with small sample sizes. Some studies lack objective measures, with results being based on self-reported data. We have supported our findings by citing references and summarizing studies in a detailed table (Table 1) for a readily accessible approach to the data.
Our goal was to look at the whole body of available literature, and as a result, we propose a need for more randomized, controlled trials to address certain limitations.
Conclusion
rTMS is a promising, noninvasive alternative therapeutic option or addition to pharmacological treatment in children and adolescents with psychiatric disorders. At this time, the majority of psychiatric research in this population is focused on adolescent depression, with limited data on other psychiatric disorders. However, current existing research has demonstrated a favorable tolerability and side effects profile of rTMS. In addition to sp/ppTMS, TBS is another emerging alternative treatment to address the feasibility in pediatric patients with reduced administration time. Ethical concerns remain a priority while investigating this application in children and adolescents. Significant research is needed to individualize and optimize the treatment in children and adolescent psychiatric disorders regarding stimulation sites and dosing parameters.
References
- Pascual-Leone A, Tormos JM, Keenan J, et al. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15(4):333–343.
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2(3):145–156.
- Buchmann J, Gierow W, Weber S, et al. Modulation of transcallosally mediated motor inhibition in children with attention deficit hyperactivity disorder (ADHD) by medication with methylphenidate (MPH). Neurosci Lett. 2006;405(1–2):14–18.
- Wu SW, Gilbert DL, Shahana N, et al. Transcranial magnetic stimulation measures in attention-deficit/hyperactivity disorder. Pediatr Neurol. 2012;47(3):177–185.
- Gilbert DL, Isaacs KM, Augusta M, et al. Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology. 2011;76(7):615–621.
- Enticott PG, Kennedy HA, Rinehart NJ, et al. GABAergic activity in autism spectrum disorders: an investigation of cortical inhibition via transcranial magnetic stimulation. Neuropharmacology. 2013;68:202–209.
- Enticott PG, Rinehart NJ, Tonge BJ, et al. A preliminary transcranial magnetic stimulation study of cortical inhibition and excitability in high-functioning autism and Asperger disorder. Dev Med Child Neurol. 2010;52(8):e179–e183.
- Rotenberg A, Horvath JC, Pascual-Leone A. Transcranial Magnetic Stimulation. Humana Press; 2014.
- Pascual-Leone A, Walsh V. Transcranial magnetic simulation. In: Toga A, Mazziotta J, eds. Brain Mapping: The Methods. Academic Press; 2002:255–290.
- Bestmann S, Baudewig J, Siebner HR, et al. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19(7):1950–1962.
- De Raedt R, Vanderhasselt MA, Baeken C. Neurostimulation as an intervention for treatment resistant depression: from research on mechanisms towards targeted neurocognitive strategies. Clin Psychol Rev. 2015;41:61–69.
- Pogarell O, Koch W, Pöpperl G, et al. Striatal dopamine release after prefrontal repetitive transcranial magnetic stimulation in major depression: preliminary results of a dynamic [123I] IBZM SPECT study. J Psychiatr Res. 2006;40(4):307–314.
- Levitt JG, Kalender G, O’Neill J, et al. Dorsolateral prefrontal γ-aminobutyric acid in patients with treatment-resistant depression after transcranial magnetic stimulation measured with magnetic resonance spectroscopy. J Psychiatry Neurosci. 2019;44(6):386–394.
- Baeken C, Lefaucheur JP, Van Schuerbeek P. The impact of accelerated high frequency rTMS on brain neurochemicals in treatment-resistant depression: insights from 1H MR spectroscopy. Clin Neurophysiol. 2017;128(9):1664–1672.
- Holtzheimer PE 3rd, McDonald WM, Mufti M, et al. Accelerated repetitive transcranial magnetic stimulation for treatment-resistant depression. Depress Anxiety. 2010;27(10):960–963.
- D’Agati D, Bloch Y, Levkovitz Y, Reti I. rTMS for adolescents: safety and efficacy considerations. Psychiatry Res. 2010;177(3):280–285.
- Quintana H. Transcranial magnetic stimulation in persons younger than the age of 18. J ECT. 2005;21(2):88–95.
- Garvey MA, Gilbert DL. Transcranial magnetic stimulation in children. Eur J Paediatr Neurol. 2004;8(1):7–19.
- Hong YH, Wu SW, Pedapati EV, et al. Safety and tolerability of theta burst stimulation vs. single and paired pulse transcranial magnetic stimulation: a comparative study of 165 pediatric subjects. Front Hum Neurosci. 2015;9:29.
- Krishnan C, Santos L, Peterson MD, Ehinger M. Safety of noninvasive brain stimulation in children and adolescents. Brain Stimul. 2015;8(1):76–87.
- Croarkin PE, Wall CA, Nakonezny PA, et al. Increased cortical excitability with prefrontal high-frequency repetitive transcranial magnetic stimulation in adolescents with treatment-resistant major depressive disorder. J Child Adolesc Psychopharmacol. 2012;22(1):56–64.
- Wall CA, Croarkin PE, Maroney-Smith MJ, et al. Magnetic resonance imaging-guided, open-label, high-frequency repetitive transcranial magnetic stimulation for adolescents with major depressive disorder. J Child Adolesc Psychopharmacol. 2016;26(7):582–589.
- Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263–1269.
- Rosenich E, Gill S, Clarke P, et al. Does rTMS reduce depressive symptoms in young people who have not responded to antidepressants? Early Interv Psychiatry. 2019;13(5):1129–1135.
- Cullen KR, Jasberg S, Nelson B, et al. Seizure induced by deep transcranial magnetic stimulation in an adolescent with depression. J Child Adolesc Psychopharmacol. 2016;26(7):637–641.
- Croarkin PE, Wall CA, King JD, et al. Pain during transcranial magnetic stimulation in youth. Innov Clin Neurosci. 2011;8(12):18–23.
- Yang XR, Kirton A, Wilkes TC, et al. Glutamate alterations associated with transcranial magnetic stimulation in youth depression: a case series. J ECT. 2014;30(3):242–247.
- Segev A, Spellun J, Bloch Y. Anxiety as a central outcome measure in an adolescent with major depressive disorder treated with repetitive transcranial magnetic stimulation. J ECT. 2014;30(4):e54–e55.
- Dhami P, Knyahnytska Y, Atluri S, et al. Feasibility and clinical effects of theta burst stimulation in youth with major depressive disorders: an open-label trial. J Affect Disord. 2019;258:66–73.
- Zhang T, Zhu J, Xu L, et al. Add-on rTMS for the acute treatment of depressive symptoms is probably more effective in adolescents than in adults: evidence from real-world clinical practice. Brain Stimul. 2019;12(1):103–109.
- MacMaster FP, Croarkin PE, Wilkes TC, et al. Repetitive transcranial magnetic stimulation in youth with treatment resistant major depression. Front Psychiatry. 2019;10:170.
- Cristancho P, Akkineni K, Constantino JN, et al. Transcranial magnetic stimulation in a 15-year-old patient with autism and comorbid depression. J ECT. 2014;30(4):e46–e47.
- Walter G, Tormos JM, Israel JA, Pascual-Leone A. Transcranial magnetic stimulation in young persons: a review of known cases. J Child Adolesc Psychopharmacol. 2001;11(1):69–75.
- Bloch Y, Grisaru N, Harel EV, et al. Repetitive transcranial magnetic stimulation in the treatment of depression in adolescents: an open-label study. J ECT. 2008;24(2):156–159.
- Oberman LM, Pascual-Leone A, Rotenberg A. Modulation of corticospinal excitability by transcranial magnetic stimulation in children and adolescents with autism spectrum disorder. Front Hum Neurosci. 2014;8:627.
- Hu SH, Wang SS, Zhang MM, et al. Repetitive transcranial magnetic stimulation-induced seizure of a patient with adolescent-onset depression: a case report and literature review. J Int Med Res. 2011;39(5):2039–2044.
- Chiramberro M, Lindberg N, Isometsä E, et al. Repetitive transcranial magnetic stimulation induced seizures in an adolescent patient with major depression: a case report. Brain Stimul. 2013;6(5):830–831.
- Sonmez AI, Kucuker MU, Lewis CP, et al. Improvement in hypersomnia with high frequency repetitive transcranial magnetic stimulation in depressed adolescents: preliminary evidence from an open-label study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;97:109763.
- Yanbin H, Zhongze L, Yunxin J, Liemin R. Repetitive transcranial magnetic stimulation-induced Charles Bonnet syndrome. Psychosomatics. 2019;60(1):101–103.
- Croarkin PE, Wall CA, Lee J. Applications of transcranial magnetic stimulation (TMS) in child and adolescent psychiatry. Int Rev Psychiatry. 2011;23(5):445–453.
- Rossi S, Hallett M, Rossini PM, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039.
- National Institute of Mental Health. Major depression. Updated Jan 2022. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Accessed 12 Mar 2021.
- Brent DA, Birmaher B. Adolescent depression. N Engl J Med. 2002;347:667–671.
- Dunn V, Goodyer IM. Longitudinal investigation into childhood-and adolescence-onset depression: psychiatric outcome in early adulthood. Br J Psychiatry. 2006;188(3):216–222.
- United States Food and Drug Administration. Revisions to Product Labeling. https://www.fda.gov/media/77404/download. Accessed 12 Mar 2021.
- Croarkin PE, Nakonezny PA, Wall CA, et al. Transcranial magnetic stimulation potentiates glutamatergic neurotransmission in depressed adolescents. Psychiatry Res Neuroimaging. 2016;247:25–33.
- United States Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/fastats/adolescent-health.htm. Accessed 12 Mar 2021.
- Croarkin PE, Nakonezny PA, Deng ZD, et al. High-frequency repetitive TMS for suicidal ideation in adolescents with depression. J Affect Disord. 2018;239:282–290.
- Wall CA, Croarkin PE, McClintock SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in adolescents with major depressive disorder. Front Psychiatry. 2013;4:165.
- Loo C, McFarquhar T, Walter G. Transcranial magnetic stimulation in adolescent depression. Australas Psychiatry. 2006;14(1):81–85.
- Mayer G, Aviram S, Walter G, et al. Long-term follow-up of adolescents with resistant depression treated with repetitive transcranial magnetic stimulation. J ECT. 2012;28(2):84–86.
- Blumberger DM, Vila-Rodriguez F, Thorpe KE et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683–1692.
- Huang YZ, Edwards MJ, Rounis E, et al. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206.
- Giam A, Chen L, Hahn L, et al. Comparing theta burst stimulation with standard left high frequency transcranial magnetic stimulation in the treatment of depression in a randomized controlled study: a preliminary comparison study. J Affect Disor Reports. 2021;5:100162.
- Li CT, Chen MH, Juan CH, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137(Pt 7):2088–2098.
- Pedapati E, DiFrancesco M, Wu S, et al. Neural correlates associated with symptom provocation in pediatric obsessive compulsive disorder after a single session of sham-controlled repetitive transcranial magnetic stimulation. Psychiatry Res. 2015;233(3):466–473.
- Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1–3):11–24.
- Aleman A, Sommer IE, Kahn RS. Efficacy of slow repetitive transcranial magnetic stimulation in the treatment of resistant auditory hallucinations in schizophrenia: a meta-analysis. J Clin Psychiatry. 2007;68(3):416–421.
- Poulet E, Haesebaert F, Saoud M, et al. Treatment of shizophrenic patients and rTMS. Psychiatr Danub. 2010;22(Suppl 1):S143–S146.
- Prikryl R, Kucerova HP. Can repetitive transcranial magnetic stimulation be considered effective treatment option for negative symptoms of schizophrenia? J ECT. 2013;29(1):67–74.
- Dlabac-de Lange JJ, Knegtering R, Aleman A. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: review and meta-analysis. J Clin Psychiatry. 2010;71(4):411–418.
- Shi C, Yu X, Cheung EF, et al. Revisiting the therapeutic effect of rTMS on negative symptoms in schizophrenia: a meta-analysis. Psychiatry Res. 2014;215(3):505–513.
- Jardri R, Lucas B, Delevoye-Turrell Y, et al. An 11-year-old boy with drug-resistant schizophrenia treated with temporo-parietal rTMS. Mol Psychiatry. 2007;12(4):320.
- Blanco-Lopez MJ, Cudeiro-Blanco J, Iglesias G, et al. A simple, repeated rTMS protocol effectively removes auditory verbal hallucinations in a single patient study. Schizophr Res. 2016;172(1–3):224–225.
- Fitzgerald PB, Benitez J, Daskalakis JZ, et al. The treatment of recurring auditory hallucinations in schizophrenia with rTMS. World J Biol Psychiatry. 2006;7(2):119–122.
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–218.
- Tikka SK, Garg S, Sinha VK, et al. Resting state dense array gamma oscillatory activity as a response marker for cerebellar-repetitive transcranial magnetic stimulation (rTMS) in schizophrenia. J ECT. 2015;31(4):258–262.
- Pedapati EV, Gilbert DL, Erickson CA, et al. Abnormal cortical plasticity in youth with autism spectrum disorder: a transcranial magnetic stimulation case-control pilot study. J Child Adolesc Psychopharmacol. 2016;26(7):625–631.
- Baruth JM, Casanova MF, El-Baz A, et al. Low-frequency repetitive transcranial magnetic stimulation (rTMS) modulates evoked-gamma frequency oscillations in autism spectrum disorder (ASD). J Neurother. 2010;14(3):179–194.
- Sokhadze E, Baruth J, Tasman A, et al. Low-frequency repetitive transcranial magnetic stimulation (rTMS) affects event-related potential measures of novelty processing in autism. Appl Psychophysiol Biofeedback. 2010;35(2):147–161.
- Gómez L, Vidal B, Maragoto C, et al. Non-invasive brain stimulation for children with autism spectrum disorders: a short-term outcome study. Behav Sci (Basel). 2017;7(3):63.
- Casanova MF, Baruth JM, El-Baz A, et al. Repetitive transcranial magnetic stimulation (rTMS) modulates event-related potential (ERP) indices of attention in autism. Transl Neurosci. 2012;3(2):170–180.
- Sokhadze EM, Baruth JM, Sears L, et al. Prefrontal neuromodulation using rTMS improves error monitoring and correction function in autism. Appl Psychophysiol Biofeedback. 2012;37(2):91–102.
- Sokhadze EM, El-Baz AS, Sears LL, et al. rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Front Syst Neurosci. 2014;8:134.
- Sokhadze EM, El-Baz AS, Tasman A, et al. Neuromodulation integrating rTMS and neurofeedback for the treatment of autism spectrum disorder: an exploratory study. Appl Psychophysiol Biofeedback. 2014;39(3–4):237–257.
- Abujadi C, Croarkin PE, Bellini BB, et al. Intermittent theta-burst transcranial magnetic stimulation for autism spectrum disorder: an open-label pilot study. Braz J Psychiatry. 2018;40(3):309–311.
- Panerai S, Tasca D, Lanuzza B, et al. Effects of repetitive transcranial magnetic stimulation in performing eye-hand integration tasks: four preliminary studies with children showing low-functioning autism. Autism. 2014;18(6):638–650.
- Ameis SH, Blumberger DM, Croarkin PE, et al. Treatment of executive function deficits in autism spectrum disorder with repetitive transcranial magnetic stimulation: a double-blind, sham-controlled, pilot trial. Brain Stimul. 2020;13(3):539–547.
- Hsu CW, Wang LJ, Lin PY. Efficacy of repetitive transcranial magnetic stimulation for Tourette syndrome: a systematic review and meta-analysis. Brain Stimul. 2018;11(5):1110–1118.
- Kwon HJ, Lim WS, Lim MH, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett. 2011;492(1):1–4.
- Le K, Liu L, Sun M, et al. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20(2):257–262.
- Kahl CK, Kirton A, Pringsheim T, et al. Bilateral transcranial magnetic stimulation of the supplementary motor area in children with Tourette syndrome. Dev Med Child Neurol. 2021;63(7):808–815.
- Mantovani A, Leckman JF, Grantz H, et al. Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of Tourette syndrome: report of two cases. Clin Neurophysiol. 2007;118(10):2314–2315.
- Singh S, Kumar S, Kumar N, Verma R. Low-frequency repetitive transcranial magnetic stimulation for treatment of Tourette syndrome: a naturalistic study with 3 months of follow-up. Indian J Psychol Med. 2018;40(5):482–486.
- Wu SW, Maloney T, Gilbert DL, et al. Functional MRI-navigated repetitive transcranial magnetic stimulation over supplementary motor area in chronic tic disorders. Brain Stimul. 2014;7(2):212–218.
- Niederhofer H. Effectiveness of the repetitive transcranical magnetic stimulation (rTMS) of 1 Hz for attention-deficit hyperactivity disorder (ADHD). Psychiatr Danub. 2008;20(1):91–92.
- Niederhofer H. Additional biological therapies for attention-deficit hyperactivity disorder: repetitive transcranical magnetic stimulation of 1 Hz helps to reduce methylphenidate. Clin Pract. 2011;2(1):e8.
- Bloch Y, Harel EV, Aviram S, et al. Positive effects of repetitive transcranial magnetic stimulation on attention in ADHD Subjects: a randomized controlled pilot study. World J Biol Psychiatry. 2010;11(5):755–758.
- Pogarell O, Koch W, Pöpperl G et al. Acute prefrontal rTMS increases striatal dopamine to a similar degree as D-amphetamine. Psychiatry Res. 2007;156(3):251–255.
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21(15):RC157.
- Moll GH, Heinrich H, Rothenberger A. [Transcranial magnetic stimulation in child and adolescent psychiatry: excitability of the motor system in tic disorders and/or attention deficit hyperactivity disorders]. Z Kinder Jugendpsychiatr Psychother. 2001;29(4):312–323.
- Weaver L, Rostain AL, Mace W, et al. Transcranial magnetic stimulation (TMS) in the treatment of attention-deficit/hyperactivity disorder in adolescents and young adults: a pilot study. J ECT. 2012;28(2):98–103.
- Gómez L, Vidal B, Morales L, et al. Low frequency repetitive transcranial magnetic stimulation in children with attention deficit/hyperactivity disorder. Preliminary results. Brain Stimul. 2014;7(5):760–762.
- Cao P, Wang L, Cheng Q et al. Changes in serum miRNA-let-7 level in children with attention deficit hyperactivity disorder treated by repetitive transcranial magnetic stimulation or atomoxetine: an exploratory trial. Psychiatry Res. 2019;274:189–194.


