 by Amne Borghol, PharmD, BCPS, BCGP; Michael Aucoin, PharmD; Ifeanyichukwu Onor, PharmD, BCPS; Dana Jamero, PharmD, BCOP; and Fadi Hawawini, DO, CMD
by Amne Borghol, PharmD, BCPS, BCGP; Michael Aucoin, PharmD; Ifeanyichukwu Onor, PharmD, BCPS; Dana Jamero, PharmD, BCOP; and Fadi Hawawini, DO, CMD
Drs. Borghol, Onor, and Jamero are with the College of Pharmacy at Xavier University of Louisiana in New Orleans, Louisiana. Dr. Aucoin is with the Woman’s Hospital in Baton Rouge, Louisiana. Dr. Hawawini is with the Geriatric and Extended Care Service at Southeast Louisiana Veterans Health Care System in New Orleans, Louisiana.
Innov Clin Neurosci. 2018;15(3–4):17–23
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Objective. The authors sought to assess the literature evidence on the efficacy of modafinil use in patients with fatigue or excessive daytime sleepiness (EDS) secondary to traumatic brain injury (TBI).
Method of Research. A literature search of Medline and PubMed was performed using the EBSCOhost database. Primary literature, observational studies, meta-analyses, case reports, and systematic reviews were assessed for content regarding modafinil and psychostimulant use in patients with TBI. Of the 23 articles collected, three randomized, controlled studies, three observational studies, one case report, and two systematic reviews gave a description of modafinil use in TBI patients.
Results and Conclusion. Modafinil is a central nervous system stimulant with well-established effectiveness in the treatment of narcolepsy and shift-work sleep disorder. There is conflicting evidence about the benefits of modafinil in the treatment of fatigue and EDS secondary to TBI. One randomized, controlled study states that modafinil does not significantly improve patient wakefulness, while another concludes that modafinil corrects EDS but not fatigue. An observational study provides evidence that modafinil increases alertness in fatigued patients with past medical history of brainstem diencephalic stroke or multiple sclerosis. Modafinil appears to have the potential to improve wakefulness in patients with TBI. A prospective, double-blinded, randomized, crossover trial of modafinil for the management of fatigue in ischemic stroke patients is currently being conducted, and further studies demonstrating consistent results are needed before making a conclusive decision.
Keywords: Traumatic brain injury, TBI, excessive daytime sleep, modafinil, fatigue, stroke, head injury
Each year in the United States, as many as 1.7 million individuals are thought to experience some form of a traumatic brain injury (TBI)- inducing event.1,2 Falls appear to be the most common cause of head injury leading to TBI (28%).3 Other medical events can also result in TBI, such as stroke, automotive accidents, and neurologic degenerative diseases. Two of the most commonly reported complications following TBI are fatigue and excessive daytime sleep (EDS), and the prevalence rate for these complications varies widely from 21 to 70 percent.4,5 A study by Gardani et al5 suggests that these sleep disturbances are common in patients with TBI undergoing rehabilitation activities and notes that clinicians often perceive sleep disorders to have an overall negative impact on rehabilitation progress. Furthermore, symptoms of fatigue/EDS are known to be associated with reduced quality of life.4
Fatigue is often defined as an overall lack of physical and/or mental energy, ordinarily stemming from inadequate nighttime sleep or excessive exercise.6,7 EDS, on the other hand, is a symptom normally present in narcolepsy, a chronic condition comprising some combination of the following four symptoms: 1) EDS, 2) cataplexy, 3) hallucinations, and 4) sleep paralysis.8 Patients suffering from this disorder will have difficulty staying awake during the day and will awaken multiple times in the middle of the night, disrupting the body’s circadian rhythm. The cause of EDS is unclear, though it is thought to be associated with decreased production of a wake-promoting hypocretin (orexin) from neuronal cells in the hypothalamus in TBI patients.9
Treatment for narcolepsy is currently focused on correcting EDS and rapid eye motion (REM) sleep patterns, and several drug classes have been used for that purpose (e.g., methylphenidates, amphetamines, modafinil, and sodium oxybate).8,10,11 The same medication classes are used for fatigue, as these disorders are often difficult to distinguish from each other. The classical psychostimulants—amphetamines and methylphenidates—work by increasing central nervous system (CNS) secretion of catecholamines (dopamine and norepinephrine).12 These stimulants are often used to treat attention deficit hyperactivity disorder (ADHD) and narcolepsy, but they might show promise in the treatment of patients with TBI, who often experience attention deficits.13 A meta-analysis conducted by Huang et al14 demonstrated statistical significance with regard to enhancing attention in patients with TBIs through 10 randomized, controlled trials utilizing methylphenidate. However, there was no notable improvement in memory or processing speed, and amphetamines were not studied.
Modafinil and its R-enantiomer, armodafinil, are categorized as nonamphetamine psychostimulants, with an unclear mechanism of action (MOA) and slightly different pharmacokinetics. Armodafinil maintains a higher plasma concentration 6 to 14 hours after administration than an equivalent dose of modafinil. Armodafinil also has a longer duration of wake-promoting activity in healthy adults. The average dose of modafinil is 200 to 400mg daily versus 150 to 250mg daily for armodafinil. Both are approved by the United States Food and Drug Administration (FDA) for the same indications (narcolepsy, shift-work sleep disorder [SWSD], and obstructive sleep apnea (OSA]).2,9,15 Additionally, a study by Tembe et al16 concluded that there was no difference in efficacy (p=0.76) or adverse events between these two CNS stimulants when given to patients with SWSD.
There is some disagreement among researchers regarding the exact MOA of modafinil and armodafinil; however, they seem to differ from the classical psychostimulants.16 One difference is that modafinil does not involve catecholamine release or reuptake.17 A literature review by Kumar 17 found that modafinil appears to decrease gamma aminobutyric acid (GABA) and increase serotonin and glutamate levels in lab mice models. In other reviews, authors have reported that modafinil might not significantly affect serotonin or GABA receptors.10,12 In an extensive review of the neurochemical actions of modafinil and its effects on cognition, Minzenberg and Carter15 reported that modafinil has a direct effect on the levels of synaptic norepinephrine (NE) and dopamine (DA) by inhibiting the NE and DA transporters, which have a direct impact on arousal and behavioral activity. They also found that modafinil seems to indirectly affect the extracellular levels of serotonin, glutamate, histamine, orexin, and GABA. Furthermore, they suggested that modafinil might be relatively more selective for cortical than for subcortical effects. The article by Elovic12 references a study using cat models that suggests that amphetamines tend to activate the striatum and large regions of the cerebral cortex (which are dopamine rich), while modafinil mostly activates the hippocampus, amygdala, and anterior hypothalamus. These findings are also supported by Kumar’s research17 with mice models.
Another difference is that modafinil is a substrate and moderate inhibitor of CYP 3A4, granting it more potential for drug-drug interactions than amphetamines.10,12 However, modafinil is relatively well-tolerated when compared to the classical psychostimulants, as it has been shown to cause less incidence of anxiety, jitteriness, hypertension, rebound effects, and severe tachycardia. The main side effects of modafinil tend to be headache (34%) and nausea (11%), which are usually mild to moderate in nature.11 The relatively low side effect profile of modafinil has led healthy patients to pursue modafinil prescriptions for the purpose of increasing performance at work or school.18 Its abuse potential has encouraged the FDA to list modafinil as a Schedule IV medication, since overdosing can result in seizures or cardiac arrhythmias—risks that are shared by the more dangerous classic psychostimulants, which are classified as Schedule II medications. Carstairs et al19 evaluated the risk potentials associated with supratherapeutic doses of modafinil, reviewing 87 cases of modafinil overdoses that were reported to the California Poison Control System electronic database over a 10-year period; no serious or death-related overdose effects were found to be associated with modafinil. This suggests that modafinil is relatively safer than other CNS stimulants.
Cost, often a largely forgotten barrier to medication access, can vary widely between the CNS stimulant drug classes. The estimated average wholesale price (AWP) is $22.07/tablet for modafinil 100mg versus $1.05/tablet for methylphenidate 10mg.20 Getting insurance companies to pay for modafinil might present a challenge to patients, depending on their coverage plan.
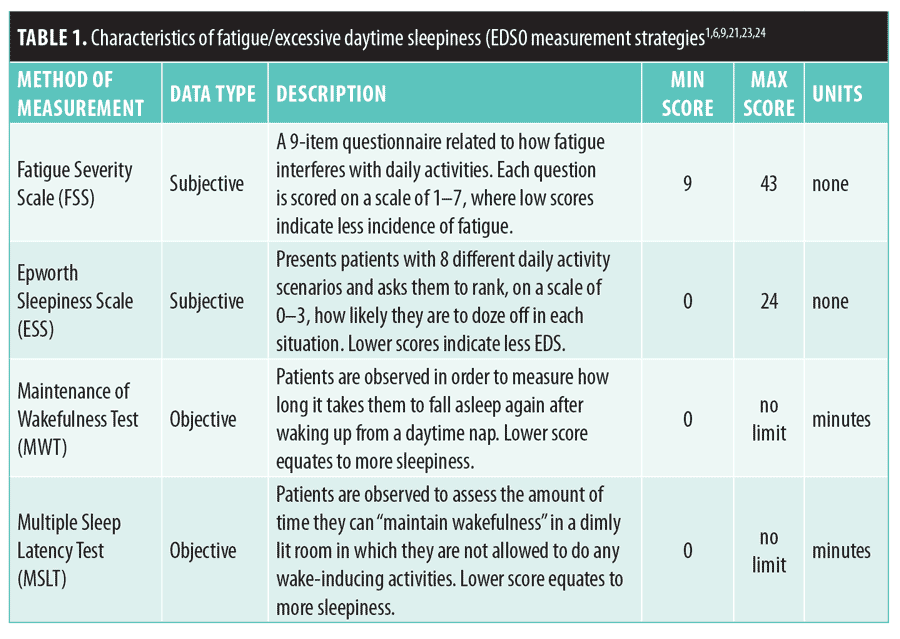
Despite its disadvantages, modafinil appears to be relatively safe compared to most other CNS stimulants and might be a valuable asset to patients with TBI who are experiencing fatigue/EDS, especially to those who have previously failed therapy with other psychostimulants. Therefore, the objective of our literature review was to determine if there is evidence of improvement in outcomes among patients with TBI taking modafinil. Among the studies reviewed, mprovement in alertness was assessed using Epworth Sleepiness Scale (ESS), Fatigue Severity Scale (FSS) Maintenance of Wakefulness Test (MWT), and/or Multiple Sleep Latency Test (MSLT) scores. The characteristics of these measurement strategies are presented in
Table 1.
Methods
Data sources. A comprehensive literature search of Medline and PubMed, using EBSCO host databases, was performed. Search terms used in combination and alone included modafinil, traumatic brain injury, stroke, fatigue, automobile accident, war injury, and excessive sleepiness. Selected articles were published between the years 2000 to 2017.
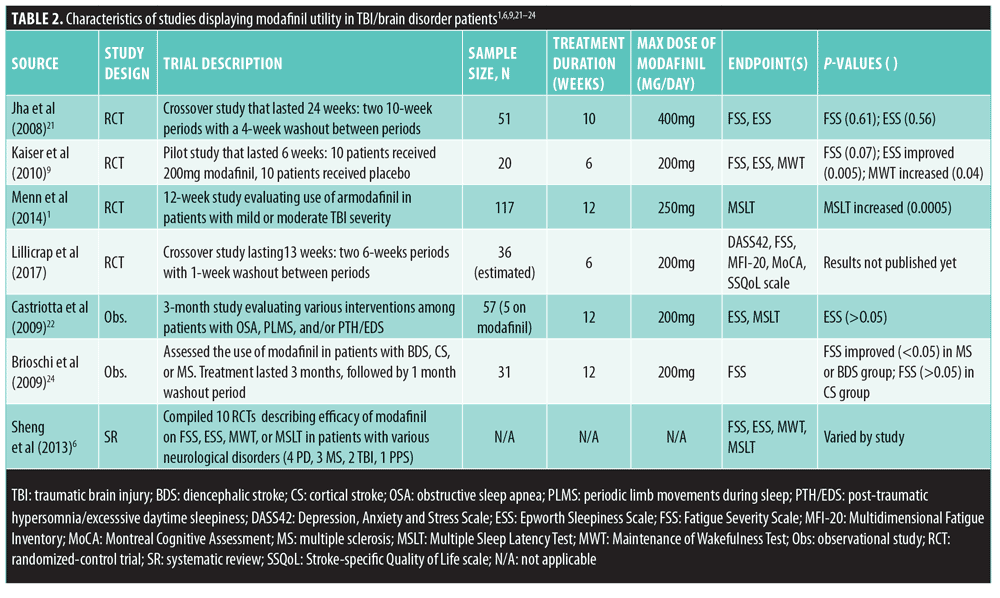
Study selection and data extraction. Sources were limited to those published in the English language and those describing modafinil and/or psychostimulant clinical trials that were conducted using human subjects. Primary literature, observational studies, meta-analyses, and systematic reviews were all examined for evidence of modafinil efficacy in patients with TBI; all causes of TBI were included. In total, eight of the 23 published articles (two systematic reviews, three randomized placebo-controlled trials, and three observational studies) and one case report describing the use of modafinil in TBI-induced EDS and fatigue provided adequate information regarding the rationale for modafinil use in the TBI population. These studies are summarized in Table 2.
Data Synthesis
Randomized, placebo-controlled trials. A study conducted by Jha et al21 describes the treatment of patients who developed fatigue and/or EDS after a TBI event that was severe enough to require inpatient rehabilitation. Exclusion criteria were TBIs caused by neurologic disorders, such as Alzheimer’s disease or stroke. Primary outcomes of the study were fatigue and EDS using FSS and ESS as measuring tools. This was a single-center, double-blind, placebo-controlled, cross-over trial during which 51 patients, all at least one year post-TBI, were randomized to receive either modafinil first, up to 400mg daily (n=27), or an equivalent amount of placebo tablets (n=24) for a period of 10 weeks. FSS and ESS scores were assessed at the end of Weeks 4 and 10. After the first 10 weeks, there was a four-week washout period where neither group was given modafinil. Then, after the four-week washout period, the groups were crossed-over to the alternate therapy for another 10 weeks. FSS and ESS scores were once again collected at the end of Weeks 4 and 10 of this 10-week period. Forty-six of the 51 initial subjects completed the entire 24-week study, with side effects being the cause of drop out among the modafinil subjects. The most frequently reported side effects in the modafinil groups were headaches (n=15) and insomnia (n=10). The trial adjusted for baseline scores and period effects (i.e., the first and second 10-week periods of the trial) and found that FSS scores for the modafinil and placebo groups differed by only a small margin of nonsignificant improvement at Week 4 (-0.5±1.88; p=0.80) and Week 10 (-1.4±2.75; p=0.61). The average change in ESS scores between modafinil and placebo was significantly greater at Week 4 (-1.2; p=0.02) but not at Week 10 (-0.5; p=0.56). Jha et al21 concluded that the variability in responses among the different subgroups of patients showed some promising results despite unclear evidence to support the use of modafinil in treating fatigue in patients with TBI. They also stated that further studies are needed to explore and further analyze the specific characteristics within the subgroups of patients with TBI that would benefit the most from using modafinil.
A randomized trial by Kaiser et al9 assessed the efficacy of modafinil on posttraumatic EDS and fatigue. This was a prospective, double-blind, randomized, placebo-controlled study that enrolled 20 patients with TBI who experienced fatigue, EDS, or both. FSS, ESS, and MWT were used to measure the outcomes of the study after a six-week treatment period with modafinil or placebo. Placebo and modafinil groups consisted of 10 subjects each; those who received modafinil were given either 100mg or 200mg daily. The results of this study showed that modafinil significantly improved ESS scores (2.3-score decrease from baseline; p=0.005) and MWT scores (8.4-minute increase from baseline; p=0.04) when compared to placebo (0.7-score increase from baseline ESS and 0.4-minute increase from baseline MWT). However, FSS scores did not significantly improve despite modafinil intervention (0.8-score decrease from baseline in modafinil group, compared to no change in the placebo group; p=0.07). Nausea, stomach pain, and arthralgia in the shoulders were reported by three patients in the modafinil group. The authors concluded that EDS, but not fatigue, is ameliorated with modafinil intervention.
Menn et al1 conducted a randomized, placebo-controlled trial to test the effectiveness and tolerability of armodafinil in patients with TBI and excessive sleepiness. The authors noted that researchers in previous studies normally chose subjects whom had suffered severe TBI, possibly explaining why modafinil was not always effective in these cases. In the Menn et al1 study, however, only patients with mild or moderate TBI were included. Primary outcomes of the study included change in mean sleep latency (assessed by the MSLT score) and percentage of responders showing improvement in wakefulness (assessed by the Clinical Global Impression of Change [CGI-C] scale). The trial lasted 12 weeks in 40 centers across the United States, followed by an optional 12-month, open-label period. Patients that met the inclusion criteria (n=117) were stratified into four different categories: placebo (n=29) or 50mg (n=30), 150mg (n=29), or 250mg (n=29) of armodafinil daily. The starting dose for all three armodafinil groups was 50mg/day, which was slowly titrated up to goal for the 150 and 250mg/day groups over a period of one week. A total of 87 patients completed the entire 12-week trial, with the highest rate of attrition seen in the 250mg/day armodafinil group. Additionally, adverse effects were highest in the group of patients receiving the largest dose of medication, with the most commonly reported side effect being headache (17%). Nausea, anxiety, and diarrhea were other commonly reported side effects of treatment. At Week 12 of the study, both the 150mg and 250mg armodafinil groups showed significantly higher MSLT scores when compared to placebo (p=0.0371 and p=0.0005, respectively). The increase in MSLT scores seemed to be directly proportional to the increase in armodafinil dose, as the 250mg group was the only statistically significant group compared to placebo at Week 4 (p=0.0152). During the final visit (either Week 12 of the study or the last post-baseline assessment), the 250mg armodafinil group had significantly higher MSLT scores from baseline compared to placebo; the lower doses studied were not significantly different at the final visit. CGI-C responder patients were defined as “much improved” or “very much improved,” and they were recorded as a percentage. Half of the patients receiving either 150mg or 250mg of armodafinil were classified as CGI-C responders by Week 4 compared to only 22 percent of placebo patients (p=0.0350 and p=0.0469 for the 150- and 250mg groups, respectively). At the end of the 12-week trial, CGI-C scores were 41 percent, 54 percent, and 48 percent for the 50-, 150-, and 250mg armodafinil groups, respectively. The placebo group had a CGI-C score of 38 percent. These scores showed no statistical significance (p>0.05). Menn et al1 concluded that armodafinil is useful in patients with mild or moderate TBI; however, they mentioned that their results were more optimistic than those seen in previous studies, and that more studies with larger samples are warranted to better clarify the role of modafinil in patients with moderate TBI.
Modafinil In Debilitating Fatigue After Stroke (MIDAS) is a Phase II, single-center, prospective, double-blinded, randomized, crossover trial of modafinil, currently being conducted in Australia, for the management of fatigue in ischemic stroke patients.22 The purpose of this study is to evaluate the efficacy of modafinil on self-reported fatigue scores and quality of life compared to placebo in patients following an ischemic stroke. Patients are being recruited from the Newcastle community and from stroke clinics in John Hunter Hospital in Australia. Participants will be randomized 1:1 to modafinil 200mg per day or placebo. The study power is 80 percent to detect a point decrease in self-reported fatigue after six weeks of modafinil treatment with a Type I error rate of 0.05. The projected sample size of this study is 36 participants in order to reach statistically significant results. The results of this ongoing study have not yet been published.
Observational studies. A prospective study by Castriotta et al23 evaluated various interventions, including modafinil therapy, in three groups with sleep disorders secondary to TBI, compared to patients without any sleep disorders, to determine if any of the interventions showed a significant improvement in patient outcomes. Patients without any sleep disorders (n=35) did not receive an intervention and were followed up in three months with neuropsychiatric testing. Patients in the first group (n=13) had obstructive sleep apnea disorder (OSA) and were treated with a continuous positive airway pressure (CPAP) device; those in the second group (n=5) had narcolepsy/EDS or post-traumatic hypersomnia (PTH) and were treated with 200mg modafinil daily; and those in the last group (n=4) suffered from periodic limb movements during sleep (PLMS) and were treated with 0.375mg of pramipexole daily. After three months, all patients underwent neuropsychiatric testing; however, they also received an ESS questionnaire and were given a MSLT Page 1 of 1 score. Patients treated with CPAP for OSA showed nonsignificant improvement in MSLT and ESS scores (p=0.66, p= 0.43, respectively). The PLMS group had normal MSLT scores from the beginning of treatment, so no improvement with pramipexole could be observed (p=0.03). Results from the use of modafinil in the EDS/PTH group showed improvements in two of the patients but overall no statistically significant differences from pre- to post-treatment; however, this group consisted of only five people, so it was difficult for the authors to draw credible conclusions.23
Another prospective, observational study conducted by Brioschi et al24 assessed modafinil effectiveness, using Fatigue Assessment Inventory (FAI) score, and tolerability among patients with histories of stroke or multiple sclerosis (MS) and self-reported fatigue. Patients were categorized into three groups: brainstem or diencephalic stroke (BDS) (n=14), cortical stroke (CS) (n=9), and MS (n=17). Participants in the study all started with 50mg of modafinil daily but dosages were titrated up to as much as 200mg per day if well-tolerated. Thirty-one patients completed the study. Headache was the most commonly reported side effect of modafinil (30% of patients) in the stroke and MS patient populations. No major side effects were reported, but minor side effects were substantial enough to cause four patients with BDS and five patients with MS to drop out before the end of the study. Data were collected from each group at baseline (T0), after three months of modafinil therapy (T1), and after one month of washout (T2). Improvement of fatigue severity (FAI score) was seen among the entire modafinil treatment cohort (p=0.006), but this was only significant in the MS and BDS groups and not the CS group. Good responders (patients with improved FAI scores) were significantly more common in the MS group (58.3%) than in the CS group (11.1% patients; p=0.04). This difference was also seen when comparing the BDS group (70%) to the CS group (11.1%; p=0.04). The authors concluded that modafinil appeared to adequately ameliorate fatigue in patients with BDS or MS and in patients who suffered a stroke and had lesions on certain areas of the brain. This led the authors to speculate that the location of the TBI might play a substantial role in determining medication selection. Additionally, patients reported improvements in daily quality of life.24
Case report. Tcheremissine and Rachal25 presented a case report of a patient with TBI who experienced benefit from using modafinil as evidenced by significant improvements in depressive symptoms and greater ability to participate in all activities of daily living. A 58-year old man who was five years post-TBI presented to the clinic with difficulty functioning in his daily routine. He reported low energy, feelings of hopelessness due to difficulty in concentration, depression, and inability to hold a job or enjoy life. His past medication history included paroxetine 30mg at bedtime, bupropion XL 300mg daily, dextroamphetamine and amphetamine (Adderall XR) 20mg daily, and zolpidem 10mg at bedtime. He reported minimal improvement from all these medications. At that time, the patient was placed on modafinil initiated at 100mg once daily and titrated to 300mg daily. The patient reported a more than 50-percent increase in his ability to participate in daily activities, with major improvements in his depressive symptoms. He was able to tolerate modafinil and did not report any side effects. The authors stated that their case provides more evidence that modafinil is a safe and efficacious adjunctive therapy for patients with TBI.
Systematic review. A systematic review and meta-analysis conducted by Sheng et al6 compiled 10 randomized, controlled trials (RCTs) describing the efficacy of modafinil as a treatment for fatigue and EDS among patients with various neurological disorders. The RCTs included four on Parkinson’s disease (PD), three on MS, two on TBI, and one on post-polio syndrome (PPS). Eight of the 10 studies collected (2 PD, 3 MS, 2 TBI, and 1 PPS) investigated the effect of modafinil on fatigue. The two PD studies showed a pooled mean FSS score of -0.22 points compared to placebo groups (p=0.66), while the three MS studies showed a pooled mean FSS score of -6.56 points compared to placebo groups (p=0.33). Neither of these findings was statistically significant. Additionally, the one PPS study showed no statistically significant difference between experiment and placebo subjects. However, the two TBI studies showed a pooled mean FSS score of -0.82 compared to placebo (p=0.02), clearly suggesting the benefit of modafinil in the setting of TBI. EDS severity was measured using ESS in four PD, two MS, two TBI, and one PPS studies. Overall mean difference between ESS scores in the four PD groups was -2.41 (p=0.004), showing clear benefit of modafinil use among patients with PD and EDS. However, ESS scores implied that modafinil was ineffective compared to placebo when treating EDS patients with either TBI or MS, or PPS (p-values not reported). Sheng et al concluded that the evidence for modafinil use in patients with PD, MS, TBI, or PPS is weak due to inconsistency of the results between trials. They noted that additional studies using larger sample sizes must be conducted and provide consistent results before a proper recommendation regarding modafinil utilization in these patient populations can be made.
One TBI study by Kaiser et al9 objectively measured sleepiness with MWT scores and found therapeutic benefit of utilizing modafinil in the treatment group (8.4±9.6; p=0.04) as compared to placebo group (0.4±6.2).
Cantor et al4 conducted a systematic review in which they collected articles that met five distinct inclusion criteria: published in English, peer-reviewed, sample size included at least 70-percent individuals with TBI, measured fatigue as a primary or secondary outcome, and involved some kind of intervention. The primary outcome of this systematic review was to identify studies that described different interventions for the management of fatigue in patients following TBI. There were only five articles that had fatigue as their primary outcome. Nineteen of the 44 articles that were fully reviewed met all the inclusion criteria. The authors found only two studies that evaluated the efficacy of modafinil in reducing fatigue and sleepiness. These two studies were also included in the meta-analysis by Sheng, et al6 Cantor et al concluded that data concerning fatigue treatment were inconsistent among the reviewed articles, that modafinil is likely ineffective for posttraumatic brain injury fatigue (PTBIF), and that further larger-scale studies are needed to evaluate fatigue treatments among different patient populations.4
Discussion
Fatigue and EDS are serious long-term complications experienced by many patients with TBI.1,4,21 However, these symptoms are often very subjective, making discovery of an adequate treatment strategy an arduous endeavor. ESS, FSS, MSLT, and MWT tests used to assess the severity of fatigue and EDS require extensive patient participation, which is not easily obtained from those who suffer from lack of energy or motivation. Prompt treatment is often needed to minimize long-term consequences of TBI; yet evidence supporting treatment modalities that result in consistent improvement in important patient parameters, such as fatigue and EDS, is substantially lacking.
The Kaiser et al9 trial concluded that modafinil was able to improve MWT scores, thereby alleviating EDS in patients with TBI; and the study conducted by Brioschi et al24 found that patients with BDS or MS and fatigure respond favorably to modafinil. However, Kaiser et al9 reported an unfavorable outcome regarding post-TBI fatigue FSS scores, and Brioschi24 reported that modafinal appeared to have no statistically significant benefit for patients with CS, as measured by ESS or FSS, compared to placebo; this implies that location of TBI might need to be determined before medication therapy can be appropriately selected, an idea supported by animal models reported by Kumar,17 Elovic,12 and Minzenberg and Carter.15 The study conducted by Jha et al21 concluded that neither EDS nor fatigue are ameliorated by the use of modafinil when compared to placebo. The lack of efficacy in the Jha et al trial is interesting because the patients were given higher dosages (up to 400mg) of modafinil than in those trials conducted by Kaiser et al9 and Brioschi et al24 (200mg modafinil/day maximum). Furthermore, the treatment period lasted longer in the Jha et al study (eight weeks) compared to the studies conducted by Kaiser et al (six weeks) or Brioschi et al (four weeks). Perhaps this difference was due to the sample size of each study, as Jha et al21 had the largest of the three trials (Jha et al, n=51; Kaiser et al, n=20; Brioschi et al, n=31). Menn et al1 had a larger sample size (n=117) than Jha et al and found therapeutic benefit of modafinil in patients with TBI. It is worth mentioning that the Menn et al study measured sleepiness objectively with MSLT scores, while Jha et al used subjective measurements of sleepiness (FSS and ESS scores). Based on these discrepancies, it might be appropriate to determine which clinical assessment (FSS, ESS, MWT, and/or MSLT) is impacted the most by modafinil when conducting future studies. The systematic review conducted by Sheng et al,6 which extensively analyzed many primary research articles, could not find consistent agreement among researchers regarding modafinil’s effectiveness on fatigue or EDS. They reported that FSS scores, but not ESS scores, showed an overall therapeutic benefit of modafinil in patients with TBI. Interestingly, FSS scores were not significantly affected in the modafinil-treated PD groups, but ESS scores were statistically significant for these same patients. Considering these results, it might be argued that the type of brain disorder (e.g., PD vs. TBI) affects the level of effectiveness (if any) of modafinil to a certain extent. It is important to note that all of the articles collected for our literature review used relatively small sample sizes, with the largest sample seen in the Menn et al1 study (n=117). Therefore, some of the examined studies might not have been powered enough to detect a statistically significant difference between treatment and placebo.
Although the results of randomized, placebo-controlled studies support the potential use of modafinil as an effective and safe treatment option for fatigue and/or EDS among patients with TBI, other studies showed no statistical improvement in either fatigue or EDS among patients with TBI when compared to placebo.21 Since fatigue and EDS are multifactorial symptoms, significant differences in the results of clinical trials are to be expected. Therefore, studies that pool larger numbers of subjects are necessary (albeit difficult to obtain from the TBI target population) in order to improve generalizability of results. Perhaps studies that focus on isolation of the actual brain injury, as suggested by Brioschi et al,24 will lead scientists to discover whom, among their patients with TBI, modafinil will work most consistently. Additionally, designing RCTs in which all participating subjects share a single cause of TBI (as opposed to multiple causes divided between subjects) might allow scientists to more accurately assess the benefit of modafinil in specific situations. Finally, the strategy for measuring sleepiness in clinical trials (e.g., FSS, ESS, MWT, and MSLT) might play an important role in the final results, particularly when using subjective measurements (e.g., FSS and ESS) in which patients might not be completely accurate in depicting their level of fatigue and sleepiness.
Other studies exist that demonstrate the potential benefit of modafinil in patients with fatigue and/or EDS. One crossover study conducted by Philip et al26 collected 27 patients with EDS (13 with narcolepsy and 14 with idiopathic hypersomnia) and randomized them to receive either 400mg/day of modafinil or placebo for five days. The subjects were then given a driving exam that challenged them to cross as few road-marked lines as possible. After a three-week washout period, the two groups switched interventions for five more days and took the driving exam again. The results showed a significant correlation between MWT scores and number of inappropriate line crossings (r= -0.41; p<0.001). This study, however, made no mention of whether any of the subjects had a history of TBI.
Adverse effects/contraindications. In addition to the inconsistency of results in the scientific literature, modafinil has some distinct disadvantages over other CNS stimulants. Modafinil is known to affect the cytochrome p450 system, particularly CYP3A4, CYP2C19, CYP2D6, and CYP2C9, a trait that is not shared with amphetamine psychostimulants, meaning the amphetamine psychostimulants will have fewer drug interactions than modafinil.10 Patients with TBI are often on medications for pain, neuropsychiatric indications, and/or anticoagulants (depending on the cause of the TBI), which makes monitoring for drug interactions with modafinil a necessity. Painful, sometimes intolerable, headaches and (rarely) seizures are other adverse effects of modafinil.12 Induruwa et al27 studied the relationship between fatigue and MS, concluding that side effects of modafinil treatment in these patients might reduce quality of life substantially, as patients with MS are often predisposed to headaches.Though modafinil carries a low risk of cardiac arrhythmias when compared to amphetamines, its adverse effects of hypertension and tachycardia might still necessitate careful monitoring in patients with heart disease, pre-existing hypertension, dyslipidemia, or diabetes.
Conclusion
Overall, scientific literature suggests that CNS stimulants such as modafinil might be useful as an adjunctive therapy among patients with TBI. Further studies with larger sample sizes and longer treatment duration are needed before making a conclusive decision regarding use of modafinial in TBI therapy.
References
- Menn SJ, Yang R, Lankford A. Armodafinil for the treatment of excessive sleepiness associated with mild or moderate closed traumatic brain injury: A 12-week, randomized, double-blind study followed by a 12-month open-label extension. J Clin Sleep Med. 2014;10(11):1181–91.
- Ouellet M-C, Beaulieu-Bonneau S, Morin CM. Sleep-wake disturbances after traumatic brain injury. Lancet Neurol. 2015;14(7):746–57.
- Viola-Saltzman M, Musleh C. Traumatic brain injury-induced sleep disorders. Neuropsychiatr Dis Treat. 2016;12:339–48.
- Cantor JB, Ashman T, Bushnik T, et al. Systematic review of interventions for fatigue after traumatic brain injury: a NIDRR traumatic brain injury model systems study. J Head Trauma Rehabil. 2014;29(6):490–7.
- Gardani M, Morfiri E, Thomson A, et al. Evaluation of sleep disorders in patients with severe traumatic brain injury during rehabilitation. Arch Phys Med Rehabil. 2015;96(9):1691–7.e3.
- Sheng P, Hou L, Wang X, et al. Efficacy of modafinil on fatigue and excessive daytime sleepiness associated with neurological disorders: a systematic review and meta-analysis. PLoS One. 2013;8(12):e81802.
- Ponsford JL, Ziino C, Parcell DL, et al. Fatigue and sleep disturbance following traumatic brain injury—their nature, causes, and potential treatments. J Head Trauma Rehabil. 2012;27(3):224–33.
- Dopp JM, Phillips BG. Sleep–Wake Disorders. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy: A Pathophysiologic Approach, 10e. New York, NY: McGraw-Hill Education; 2017.
- Kaiser PR, Valko PO, Werth E, et al. Modafinil ameliorates excessive daytime sleepiness after traumatic brain injury. Neurology. 2010;75(20):1780–5.
- Schwartz JRL. Modafinil in the treatment of excessive sleepiness. Drug Des Devel Ther. 2009;2:71–85.
- Cheng J, Groninger H. Modafinil #259. J Palliat Med. 2012;15(12):1388–9.
- Elovic E. Use of provigil for underarousal following TBI. J Head Trauma Rehabil. 2000;15(4):1068–71.
- Jenkins PO, Mehta MA, Sharp DJ. Catecholamines and cognition after traumatic brain injury. Brain. 2016;139(9):2345–71.
- Huang C-H, Huang C-C, Sun C-K, et al. Methylphenidate on cognitive improvement in patients with traumatic brain injury: a meta-analysis. Curr Neuropharmacol. 2016;14(3):272–81.
- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33(7):1477–502.
- Tembe DV, Dhavale A, Desai H, et al. Armodafinil versus modafinil in patients of excessive sleepiness associated with shift work sleep disorder: A randomized double blind multicentric clinical trial. Neurol Res Int. 2011;2011.
- Kumar R. Approved and investigational uses of modafinil: An evidence-based review. Drugs. 2008;68(13):1803–39.
- Kim D. Practical use and risk of modafinil, a novel waking drug. Environ Health Toxicol. 2012;27:e2012007.
- Carstairs SD, Urquhart A, Hoffman J, et al. A retrospective review of supratherapeutic modafinil exposures. J Med Toxicol. 2010;6(3):307–10.
- Red Book Online{®} System (electronic version). Greenwood Village, Colorado: Truven Health Analytics; 2017.
- Jha A, Weintraub A, Allshouse A, et al. A randomized trial of modafinil for the treatment of fatigue and excessive daytime sleepiness in individuals with chronic traumatic brain injury. J Head Trauma Rehabil. 2008;23(1):52–63.
- Lillicrap T, Krishnamurthy V, Attia J, et al. Modafinil in debilitating fatigue after stroke (MIDAS): study protocol for a randomised, double-blinded, placebo-controlled, crossover trial. Trials. 2016;17(1):410.
- Castriotta RJ, Atanasov S, Wilde MC, et al. Treatment of sleep disorders after traumatic brain injury. J Clin sleep Med. 2009;5(2):137–44.
- Brioschi A, Gramigna S, Werth E, et al. Effect of modafinil on subjective fatigue in multiple sclerosis and stroke patients. Eur Neurol. 2009;62(4):243–9.
- Tcheremissine OV, Rachal JC. Modafinil augmentation therapy in patient with traumatic brain injury. Innov Clin Neurosci. 2017;14(3–4):11.
- Philip P, Chaufton C, Taillard J, et al. Modafinil improves real driving performance in patients with hypersomnia: a randomized double-blind placebo-controlled crossover clinical trial. Sleep. 2014;37(3):483–7.
- Induruwa I, Constantinescu CS, Gran B. Fatigue in multiple sclerosis—a brief review. J Neurol Sci. 2012;323(1–2):9–15.





