by ISCTM Suicidal Ideation and Behavior Assessment Workgroup: Phillip B. Chappell, MD; Atul R. Mahableshwarkar, MD; Larry D. Alphs, MD, PhD; Mark E. Bangs, MD; Adam Butler, BA; Sarah J. Dubrava, MS; John H. Greist, MD; William R. Lenderking, PhD; James C. Mundt, PhD; and Michelle Stewart, PhD
by ISCTM Suicidal Ideation and Behavior Assessment Workgroup: Phillip B. Chappell, MD; Atul R. Mahableshwarkar, MD; Larry D. Alphs, MD, PhD; Mark E. Bangs, MD; Adam Butler, BA; Sarah J. Dubrava, MS; John H. Greist, MD; William R. Lenderking, PhD; James C. Mundt, PhD; and Michelle Stewart, PhD
Dr. Chappell is with Pfizer, Inc., Groton, Connecticut; Dr. Mahableshwarkar is with Takeda Pharmaceutical Company, Deerfield, Illinois; Dr. Alphs is with Janssen Scientific Affairs, Titusville, New Jersey; Dr. Bangs is with Eli Lilly and Company, Indianapolis, Indiana; Mr. Butler is with Bracket Global, Wayne, Pennsylvania; Ms. DuBrava is with Pfizer, Inc., Groton, Connecticut; Dr. Greist is Clinical Adjunct Professor of Psychiatry at University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin; Dr. Lenderking is with Evidera, Bethesda, Maryland; Dr. Mundt is with Center for Telepsychology, Madison, Wisconsin; and Dr. Stewart is with Pfizer, Groton, Connecticut.
Innov Clin Neurosci. 2014;11(9–10):14–22
Funding: There was no funding for the development and writing of this article.
Financial Disclosures: Dr. Chappell is a full-time employee of Pfizer, Inc., Groton, Connecticut; Dr. Mahableshwarker is a full-time employee of Takeda Pharmaceutical Company, Deerfield, Illinois; Dr. Alphs is employed by Janssen Scientific Affairs, Titusville, New Jersey, and is a stockholder of Johnson & Johnson; Dr. Bangs has no conflicts of interest relevant to the content of this article; Mr. Butler is an employee of Bracket Global, Wayne, Pennsylvania; Ms. DuBrava is an employee of Pfizer, Inc. and holds stock in the company; Dr. Greist is a co-developer and coauthor of the electronic Columbia-Suicide Severity Rating Scale (eC-SSRS) and a principal at Healthcare Technology Systems, Inc., which receives royalties from eC-SSRS use; Dr. Lenderking is a stockholder of Pfizer, Inc., Groton, Connecticut; Dr. Mundt is a stockholder in Healthcare Technology Systems, which receives royalties from ERT for the development and validation of the eC-SSRS. ERT supported the meta-analysis of the reported data and preparation of the manuscript for publication submission; Dr. Stewart is a full-time employee of Pfizer, Inc., Groton, Connecticut.
Key words: Prospective assessment of suicidal ideation and behavior in industry studies, C-SSRS in industry studies
Abstract: Objective: To survey the current approaches of clinical trial sponsors in prospective suicidal ideation and behavior assessments and challenges encountered. Design: An internet-based survey. Setting: Inclusion of prospective assessments of suicidal ideation and behavior in industry-sponsored clinical studies were required following the release of the September 2010 United States Federal Drug Administration draft guidance. The International Society for CNS Clinical Trials and Methodology Suicidal Ideation and Behavior Assessment Workgroup conducted an online survey to understand industry practices and experiences in implementing suicidal ideation and behavior assessments in clinical trials. Participants: The survey was sent to 1,447 industry employees at 178 pharmaceutical companies. A total of 89 evaluable responses, representing 39 companies, were obtained. Measurements: A 30-item internet survey was developed asking about potential challenges and issues in implementing prospective suicidal ideation and behavior assessments. Results: Common factors in deciding whether to include suicidal ideation and behavior assessments in a clinical trial were psychiatric or neurologic drug product (95%); central nervous system activity (78%); disease (74%) and patient population (71%); and regulatory announcements and policies (74%). The most common challenges in implementing suicidal ideation and behavior assessments included cross-cultural differences in acceptance of SIB assessments (40%); obtaining adequate baseline history (36.8%); obtaining translations (35%); investigator/rater discomfort with asking about suicidal ideation and behavior (32%); and inadequate training of raters to administer suicidal ideation and behavior ratings (30%). Conclusion: Among sponsors surveyed, the implementation rate of suicidal ideation and behavior assessment in central nervous systems studies is very high. Most have used the Columbia-Suicide Severity Rating Scale. Challenges regarding standardization of retrospective assessment timeframes and differing approaches to summarizing and analyzing suicidal ideation and behavior-related study data were frequently reported. These results suggest that inconsistent reports of suicidal ideation and behavior within study datasets may occur and that integration of data across studies remains a concern.
Introduction
In September 2010, the United States Food and Drug Administration (FDA) issued an initial draft guidance requiring prospective assessment of suicidal ideation and behavior (SIB) in clinical trials of central nervous system (CNS) active compounds under development for psychiatric and neurologic indications and in certain other conditions (e.g., obesity, smoking cessation).[1] The guidance lists two objectives for introducing these systematic assessments into clinical trials: 1) to better identify patients in clinical trials who experience SIB to ensure they are adequately treated and 2) to enable more complete and timely collection of data on SIB events during trial conduct. Following the appearance of the guidance and its revision in August 2012,[2] prospective assessments of SIB were broadly implemented in clinical studies spanning a wide range of indications, patient populations, geographic regions, and cultures.
To better understand the impact of this development on the clinical trial process, the International Society of CNS Clinical Trials and Methodology (ISCTM) formed a work group comprising stakeholders involved with clinical trials (investigators, raters, sponsors, vendors) to survey current approaches to SIB assessment following the issuance of the FDA draft guidance. In the summer of 2011, the ISCTM SIB Assessment Workgroup (ISAW) conducted an initial online survey of clinical trial site experiences and attitudes toward SIB data collection.[3] A total of 972 evaluable responses were collected, and the majority of respondents had personally conducted SIB assessments. The results revealed that although SIB assessments were readily incorporated into clinical trials and improved patient safety, there were continuing challenges for sites in certain areas such as obtaining an accurate baseline lifetime history, training in administration of the SIB rating scale, and assessment of SIB in cognitively impaired populations.[3]
To complement the previous survey of clinical trial sites, in August 2013, the ISAW conducted an online survey regarding pharmaceutical sponsor experience and practices in the conduct of prospective SIB assessments in clinical trials. The survey addressed topics regarding the types of studies that typically monitor SIB, factors considered in deciding if a study should include SIB assessments, which assessment tools or rating instruments were used, how SIB screening and baseline assessments are done, how subjects reporting active SIB during a study are managed, and what challenges companies encounter implementing SIB assessments in their studies.
Methods
Survey questionnaire development. Potential challenges and issues encountered by pharmaceutical sponsors in implementing SIB assessments in industry clinical trials were identified in discussion with stakeholders within and outside the ISAW. Based on these discussions, 30 items were developed for inclusion in the survey. Four questions elicited demographic and background information on the respondent, including type of training, type of company worked for (large, medium, or small pharmaceutical company or biotech), role in the company, and years of experience in industry. Respondents were asked whether they were involved with the implementation of SIB assessments in clinical trials at their company. A “final draft” version of the survey was piloted with several non-workgroup participants from pharmaceutical and biotech companies prior to being finalized and implemented online using Survey Monkey (Supplemental Document 1*).
Sample identification. A list of email addresses was assembled from the ISCTM membership mailing list and from a contact mailing list maintained by a vendor that provides clinical trial scientific services to pharmaceutical companies. The survey was sent to 1,447 industry employees at 178 pharmaceutical and biotech companies. Representatives from contract research organizations (CROs), vendors, or academic and government institutions were not included in the survey. Data were collected from August 15, 2013, to September 20, 2013.
A cover email accompanying the survey invitation encouraged respondents to discuss the survey questions with colleagues, if necessary, to provide information that best represented their company’s practices and experiences with SIB assessments in clinical trials.
Data analysis. Continuous variables were summarized using descriptive statistics. Categorical variables were summarized using frequency and percent tabulations. The number of respondents who answered each item was used as the denominator in calculating percentages because a different number of respondents answered each of the questions.
Results
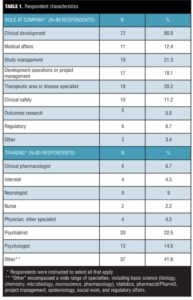
A total of 132 responses from 50 companies were collected over five weeks for a response rate of 9.1 percent. Respondents with no involvement in SIB assessments in clinical trials at their company were excluded from further analysis, leaving a total of 89 evaluable responses, which represented 39 companies (for an evaluable response rate of 6.1%). About half (52%, 45/87) of the respondents identified themselves as working at a large pharmaceutical company, and about a third (35%, 30/87) at mid-sized companies, while the remainder reported they worked at a small pharmaceutical company or biotech.
About 40 percent (36/89) of respondents were physicians, with the largest specialty (22.5%, 20/89) represented by psychiatrists (Table 1). Thirty-seven of the 89 respondents (41.6%) described their background training as “Other,” and in open text comments listed a wide range of disciplines that included basic science, statistics, clinical pharmacology/pharmacist training, project management, epidemiology, social work, and regulatory affairs. In terms of the roles they played in their companies, most respondents (80.9%, 72/89) described themselves as working in clinical development (Table 1). The majority (87.5%, 77/88) reported having worked in the industry for more than 10 years.
Factors considered by companies in deciding to include SIB assessments in studies. The most common factors taken into account by pharmaceutical or biotech companies in deciding whether prospective SIB assessments should be included in clinical trials were whether the compound under study was a psychiatric or neurologic drug product; whether it was CNS active; the disease and patient population under study; and regulatory announcements and policies. Nearly half of respondents also indicated that evidence of SIB adverse effects in other drugs of the same class and the occurrence of CNS side effects in Phase 1 studies were important considerations.
Patients’ ability to understand the wording and provide meaningful responses to SIB assessments was identified as an important factor by about 40 percent of the 83 respondents who answered the question. About 60 percent (51/83) of the respondents identified six or more important factors, and 35 percent (29/83) endorsed 10 or more factors (Supplemental Table 1*).
CNS and non-CNS indications for which companies have included SIB assessments in clinical trials. Respondents reported that their companies had included SIB assessments in a broad-range of psychiatric and neurologic disorders. The most common indications, endorsed by 20 percent or more of respondents (N=81), included schizophrenia, depression, bipolar disorder, Alzheimer’s disease or other dementia, anxiety, attention deficit hyperactivity disorder (ADHD), pain, and mild cognitive impairment (MCI). Eight respondents (9.9%) indicated their company had included SIB assessments in studies specific to SIB indications. Whether these eight responses reflect SIB assessment to monitor patient safety and/or as an efficacy outcome measure was not elicited by the survey (Supplemental Table 2*).
In line with specific requirements in the FDA guidance,[1, 2] smoking cessation and obesity were among the non-CNS indications endorsed by the survey respondents as including SIB assessments in clinical studies. The most common non-CNS indications reported by the respondents were fibromyalgia (40.6%, 13/32) and insomnia or other sleep disorders (31.3%, 10/32) (Supplemental Table 3*).
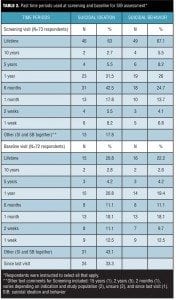
Past time periods used for SIB assessments at screening and baseline. Almost all (99%) of the respondents indicated that SIB assessments were performed at both screening and baseline visits. The most commonly endorsed look-back periods used at screening visits were lifetime (63%, 46/73 for SI; 67.1%, 49/73 for SB); one year (31.5%, 23/73 for SI; 26%, 19/73 for SB); six months (42.5%, 31/73 for SI; 24.7%, 18/73 for SB); and one month (17.8%, 13/73 for SI; 13.7%, 10/73 for SB) (Table 2). Generally, respondents indicated the use of longer look-back periods for assessment of SB compared to SI at the screening visit.
The most commonly reported look-back period used at baseline visits for assessing SIB was “Other” (43.1%, 31/72) (Table 2). Among the 31 “Other” responses, 24 respondents (33.3%, 24/72) reported using the interval since the screening visit. Aside from the screening-to-baseline interval, the next most frequently reported time periods included lifetime (20.8%, 15/72 for SI; 22.2%, 16/72 for SB), one year (20.8%, 15/72 for SI; 19.4%, 14/72 for SB), one month (18.1%, 13/72 for both SI and SB), six months (11.1%, 8/72 for both SI and SB), and two weeks (11.1%, 8/72 for SI; 9.7%, 7/72 for SB).
Survey respondents were also asked to indicate what past time periods were used at the screening visit to determine if the subject is at risk of suicide and should be excluded from the study. The most commonly endorsed past time periods for subject exclusion were one year (33.8%, 22/65 for SI; 32.3%, 21/65 for SB), six months (26.2%, 17/65 for SI; 23.1%, 15/65 for SB), lifetime (21.5%, 14/65 for SI; 29.2%, 19/65 for SB), and one month (15.4%, 10/65 for SI; 13.8%, 9/65 for SB). Seventeen respondents (26.2%, 17/65 of the total) endorsed the “Other” option. In free-text comments, time periods of two years (5), three months (2), and two months (1) were noted, and four respondents indicated that the time period varied by study design and/or treatment indication.
SIB assessment instruments used by the companies. The most commonly used SIB instrument was the C-SSRS.[4,5] About 95 percent of respondents indicated their company used the C-SSRS for screening (64/76) and baseline (63/67) assessment of SIB, as well as for tracking the emergence of SIB during the course of clinical trials. About 18 percent (12/67) of respondents indicated their company had used the interactive voice response (IVR) version of the C-SSRS (the eC-SSRS).[6, 7]
Additional instruments used included the Sheehan Suicide Severity Tracking Scale (S-STS)[8] (about 20%, 14/67)) and the InterSept Scale for Suicide Thinking (ISST)[9] (about 10%, 7/67). Twenty-two percent (15/67), 10.4 percent (7/67), and 9.0 percent (6/67) of respondents reported their company had used the Suicidality Module of the Mini Neuropsychiatric Interview10 for screening, baseline, and post-baseline assessments, respectively.
Of note, about a fourth of respondents reported using the single suicide item of the Hamilton Rating Scale for Depression (HAM-D)[11] and a third reported using the suicide item of the Montgomery–Åsberg Depression Rating Scale (MADRS)[12] for screening/baseline SIB assessment; they also reported using these single-item ratings to track SIB emergence during clinical trials. A smaller percentage of respondents (?10%) reported having used the suicide item of the Patient Health Questionnaire (PHQ-9),[13] the Inventory for Depressive Symptomatology (IDS)/Quick Inventory of Depressive Symptomatology (QIDS),[14] and/or the Children’s Depression Rating Scale (CDRS).[15]
About 60 percent (39/67) of respondents reported their company had used more than one SIB assessment instrument (ranging from 2 to >5). Due to the wording of the survey question, it is not possible to tell if these responses refer to current or past practices of the companies.
SIB instruments used in special populations. The use of SIB assessments in pediatric populations was not uncommon: 38.1 percent (24/63) and 44.4 percent (28/63) of respondents reported their company had obtained SIB assessments in children younger than 12 years of age and in adolescents aged 12 to 17 years, respectively.
The most commonly used instruments in children under the age of 12 years were the pediatric version of the C-SSRS[16] (41.7%, 10/24), the adolescent/adult version of the C-SSRS (37.5%, 9/24), the suicide ideation item of the CDRS[15] (33.3%, 8/24), and the suicidality module of the Mini-Kid Neuropsychiatric Interview (16.7%, 4/24).[10] In free-text comments, two respondents indicated their company had used the IVR version of the C-SSRS in clinical studies of children under 12 years of age, with parents/caregivers answering the eC-SSRS questions for younger children. One respondent reported having used the Reynolds Suicidal Ideation Questionnaire.[17]
The C-SSRS was also the most commonly used instrument in adolescents 12 to 17 years of age, with 56.7 percent (17/30) of respondents reporting use of the adolescent/adult version of the C-SSRS and 33.3 percent (10/30) reporting use of the pediatric version of the C-SSRS. Two respondents also reported use of the IVR version of the C-SSRS in adolescent clinical trials. The survey respondents reported only limited use of other instruments in this age group (e.g., only 10% (3/30) reported use of the S-STS or the Suicidality Module of the Mini), and a small number of respondents reported their companies had employed the single suicide items of the HAM-D, MADRS, PHQ-9, or CDRS.
Respondents were also asked if their company had included SIB assessments in clinical trials of patients with cognitive impairment. Forty-one of 69 respondents answered affirmatively, and reported that the most commonly used instrument was the C-SSRS (18 respondents); use of the pediatric version of the C-SSRS and the S-STS was reported by seven and four respondents, respectively. Thirty-four of these 41 respondents also reported that their company had included SIB assessments in clinical trials of either Alzheimer’s disease or dementia (N=27), MCI (N=15), autism (N=9), or Down’s syndrome (N=1).
Management of subjects reporting active SIB in clinical trials. The majority of respondents (52.5%, 31/59) who answered the question indicated that subjects who reported active SIB during the course of a clinical study were either kept in the study and referred to a mental health clinician for a risk assessment to determine if it was safe for the subject to continue in the study, or were discontinued from the study and referred to a mental health expert for evaluation (44.1%, 26/59). No one reported that subjects were continued in the study without further assessment; two respondents to this question indicated that subjects were discontinued from the study without a specific referral for further evaluation.
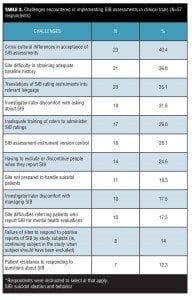
A substantial minority of respondents reported (Table 3) they had experienced challenges with study sites not being prepared to handle suicidal patients (19.3%, 11/57) and site difficulties in referring patients who are actively suicidal for mental health evaluations (17.5%, 10/57).
Other sources of data used in SIB assessments. The use of sources of data in addition to direct assessment of patients in clinical trials was reported by the majority (88%, 52/59) of respondents, who answered the question, with 67.8 percent (40/59) of respondents reporting more than one additional source of data had been used. Only 12 percent indicated no external sources of SIB data were sought beyond direct SIB assessments.
Other sources of SIB data used in assessing suicidal risk of patients in clinical trials included information provided by the patient’s family (72.9%, 43/59), the referring physician or medical care provider (64.4%, 38/59), assisted living or nursing home staff (30.5%, 18/59), and other informants, such as friends or neighbors (18.6%, 11/59). More than half of the respondents (52.5%, 31/59) also indicated that medical, psychiatric, and coroner records had been used.
Approach to the statistical analysis of SIB data. Among the respondents who answered the question, about half (51.5%, 34/ 66) reported their company prepared separate study-level summaries each of SI and SB data, and about a third (30.3%, 20/66) reported their company summarized study-level SI and SB data together. A small fraction (10.6%, 7/66) indicated their company did not have a standard approach for summarizing study-level data.
About 40 percent (27/66) of respondents reported that, in addition to prospective assessment of SIB, their company also performed retrospective, post-hoc analyses of potential suicide-related adverse events using computerized searches of verbatim adverse event reports and submission of narratives for external adjudication. The survey did not clarify whether companies were performing post-hoc, retrospective analyses of data from studies that also included prospective assessments of SIB.
Implementation challenges. As shown in Table 3, top challenges (identified by at least 20% of the 57 respondents to this item) for implementing SIB assessments in clinical trials included cross-cultural differences in acceptance of SIB assessments (40.4%, 23/57); site difficulty in obtaining adequate baseline history ( 36.8%, 21/57); translation of SIB assessments to relevant languages (35.1%, 20/57); investigator/rater discomfort asking about SIB (31.6%, 18/57); inadequate rater training to administer SIB assessments (29.8%, 17/57); version control of SIB assessment instruments (28.1%, 16/57); and having to exclude/discontinue patients if they report SIB (24.6%, 14/57). The majority of respondents (80.7%, 46/57) reported their company had experienced multiple challenges in implementing SIB assessments in clinical trials (range from 2 to >5 different types of challenges).
Discussion
The results of this survey provide initial evidence supporting the view that prospective assessment of SIB has been widely incorporated in industry-sponsored clinical trials since the appearance of the FDA draft guidance in 2010.[1] Not surprisingly, the most common indications for which monitoring has been done include psychiatric and neurocognitive disorders, but monitoring has also been extended to a range of other neurologic and pain conditions, including studies in stroke victims and patients with neurodegenerative disorders such as amyotrophic lateral sclerosis and Parkinson’s disease.
The C-SSRS[5,6] was identified by the survey as the most commonly used instrument for prospective assessment of SIB in industry-sponsored clinical trials. This is in line with its endorsement as an acceptable scale in both the 2010 and 2012 FDA draft guidance.[1, 2] A significant number of respondents also reported using the S-STS and other scales; however, the survey did not elicit comparative information on sponsor experiences with the different scales, which would have been of interest.
Most importantly, the results of this survey show that a broad range of time periods is being utilized for the assessment of SIB in subjects considered for clinical studies. This diversity may be related to the screening and baseline use of the SIB assessment. Specifically, it may relate to whether the assessment is used to include or exclude subjects from the study and/or whether it is being used to assign a current baseline SIB status to the subject for use in analysis of the study data for emergence of new SIB. There appears to be little consistency in choice of past time periods for screening and baseline assessments; nor is it clear how look-back periods have been derived.
The lack of consistency in assessment periods used by different companies suggests substantial uncertainty about what is the appropriate past time period for determining subject-level suicide risk at study entry. Some confusion may derive, in part, from the August 2012 FDA draft guidance that baseline SIB evaluation should include an assessment of lifetime SIB.[2] Use of a lifetime assessment for determining the baseline SIB status of a subject may be problematic, as SIB events of the distant past may not be relevant to current clinical states. Inclusion of remote events may also lead to inflation of reported baseline rates of SIB. Further research is needed to identify optimal look-back periods for use at screening to identify subjects at increased risk of suicide, who may require increased surveillance, and for use at baseline to assign current SIB status for detection of treatment emergent SIB. While the optimal look-back periods may vary across indications, standardization of assessment time periods at the screening and baseline visits would facilitate data aggregation across multiple studies within a development program to support meta-analysis of SIB data as well as across different programs from the same or different companies.
The variability reported by survey respondents regarding the approach different companies have taken to summarize study-level SIB data also suggests there is confusion among sponsors regarding best practices for analyzing and reporting clinical trial SIB data. Implementation of prospective assessment of SIB in clinical trials was anticipated to make retrospective analysis of potential suicide-related adverse event data unnecessary.[1,2] However, the results of the current survey suggest that some companies may continue to utilize both approaches to the analysis of their SIB data. Although cross-industry efforts have been undertaken to develop a consensus position regarding the best methods for analyzing and reporting study-level SIB data, the impact of these recommendations across the industry, to date, appears to be limited.[18]
Numerous challenges for implementing SIB in clinical trials were identified. Interestingly, cross cultural differences in the acceptance of SIB assessments was the most commonly reported challenge by sponsors. This is in contrast to the earlier ISAW survey of study sites that did not identify this as a major issue.[3] Similar to findings from the previous survey, difficulty obtaining translations of SIB instruments into relevant languages was a challenge for a substantial proportion (35.1%) of respondents, despite the reported availability of a wide range of translations of the C-SSRS (http://www.cssrs.columbia.edu/translations_cssrs.html). These findings could have implications for the quality of SIB data collected in global studies, which are conducted in many regions and different cultures across the world. Such concerns may require development of more culturally sensitive SIB assessment tools, as well as culturally specific rater training materials and methods. Availability of accurate translations of SIB tools is a necessary first step, but alone may not be sufficient to address cultural sensitivities and resistances around revelations of suicidal thoughts and behaviors.
The second most common challenge identified by this survey was site difficulty in obtaining an adequate baseline SIB history. This issue was also reported as a major challenge by site investigators in the prior survey of clinical study sites.[3] Taken together, the results of these two surveys suggest there is a persistent problem in this area. Given the critical importance of the baseline assessment in determining the emergence of new or worsening SIB during the course of a treatment study, further research is urgently needed to better understand the accuracy of baseline SIB assessments being acquired in clinical studies and, if necessary, to identify methods to improve the accuracy of the information being collected and reported. More consistent use of multiple sources of information may be one means of improving the validity of baseline SIB assessments.
Overall the survey results suggest that the FDA guidance does appear to be working as it was intended: respondents indicated that subjects experiencing SIB during an ongoing study are being identified and are receiving follow-up evaluation, whether they continue in the trial or not, and prospective data on the occurrence of SIB in clinical trials are being collected for further study. Challenges were identified, however, with reports that some study sites are ill prepared to handle subjects experiencing SIB, as well as obtaining referrals for mental health assessments. Although these challenges were reported by a minority of respondents (<20%), they are important to note, as they potentially impact patient safety.
The major limitations of this study include the relatively low response rate and the possibility of variability in response conditions (e.g., in some cases, respondents may have consulted with colleagues as encouraged by the survey instructions, but in other cases they may have not done so). Taken together, these factors may have resulted in unrecognized bias, which could limit the generalizability of the results. Although the survey instructions encouraged respondents to discuss the questions with colleagues at their company and provide a single representative response to the survey, on average there were about two responses for each company represented in the survey sample (with a range of 1 to 6). The study results did not differentiate respondents who obtained broader input from those who did not. Issues of this nature may be inevitable, however, in surveys of large companies where different business units and functional lines may be independently involved with SIB assessments, and there may or may not be a standardized approach to how SIB assessments are conducted and the data analyzed.
An additional limitation of this online study is its reliance on self-reported data, which cannot be objectively verified. Finally, in asking about SIB assessment instruments and practices used by the companies, the survey did not distinguish between current and past practices; doing so would have provided additional useful background on the evolution of company choices in both these areas.
Conclusion
In summary, the results of the present survey support the view that many pharmaceutical sponsors have attempted to implement FDA recommendations for inclusion of prospective SIB assessments in clinical trials. However, sponsors continue to face numerous challenges, especially in global studies, which could impact data quality. Consistent with the findings of the previous survey of study sites,3 site difficulty in obtaining an adequate baseline SIB history is a continuing challenge. There also are continuing important questions regarding standardization of retrospective assessment timeframes and differing approaches to summarizing and analyzing SIB-related study data. Taken together, these results suggest that inconsistent reports of SIB within study datasets may occur and that integration of data across studies and development programs remains a concern. Likewise, some sites would benefit from assistance in developing appropriate patient evaluation and treatment referral networks to respond to reported SIB during trial participation.
In the years since the appearance of the initial FDA guidance for industry on prospective assessment of SIB in clinical trials,[1] pharmaceutical companies have made significant efforts to comply with the FDA’s recommendations.[3,18] Results of the current survey show these efforts have identified a number of operational, technical, and statistical challenges that may impact the quality of SIB-related clinical trial data and compromise the goals of the FDA guidance. Based on this experience, we believe re-convening key stakeholders from academia, industry, and the FDA to review what has been learned about implementation of prospective SIB assessments in clinical trials would be beneficial. Such a meeting could help identify potential solutions to persistent challenges. Such a meeting could also help build an informed consensus regarding standardization of the information and risk factors affecting SIB that need to be collected, as well as operational standards regarding the methods for collecting, analyzing, interpreting, managing, and reporting SIB-related study data.
*Supplement Document and tables
Click here to access Supplemental Document 1.
Click here to access Supplemental Tables 1–4.
Acknowledgments
The authors gratefully acknowledge the assistance of Dr. Jill Harkavy-Friedman of the American Foundation for Suicide Prevention in the design of the survey and preparation of this manuscript and of ISCTM staff members Carlotta McKee and Mary Bea Harding in the conduct of the survey and operational support in preparation of the manuscript.
References
1. United States Food and Drug Administration, United States Department of Health and Human Services. Guidance for Industry: Suicidality: Prospective Assessment of Occurrence in Clinical Trials, Draft Guidance. September 2010. https://www.federalregister.gov/articles/2010/09/09/2010-22404/draft-guidance-for-industry-on-suicidality-prospective-assessment-of-occurrence-in-clinical-trials. Accessed October 1, 2014.
2. United States Food and Drug Administration, United States Department of Health and Human Services. Guidance for Industry: Suicidality: Prospective Assessment of Occurrence in Clinical Trials, Draft Guidance. August 2012. Revision 1. http://www.fda.gov/downloads/Drugs/Guidances/UCM225130.pdf. Accessed October 1, 2014.
3. Stewart M, Butler A, Alphs L, et al. Study site experiences and attitudes towards prospective assessments of suicidal ideation and behavior in clinical trials: Results of an internet-based survey. Innov Clin Neurosci. 2013;10(5–6 Suppl A):20S–28S
4. Posner K, Oquendo MA, Gould M, et al. Columbia Classification Algorithm of Suicide Assessment (C–CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164:1035–1043
5. Posner KP, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011; 168:1266–1277
6. Mundt JC, Greist JH, Gelenberg AJ, et al. Feasibility and validation of a computer-automated Columbia-Suicide Severity Rating Scale using interactive voice response technology. J Psych Res. 2010; 44(16): 1224–1228.
7. Mundt JC, Greist JH, Jefferson JW, et al. Prediction of suicidal behavior in clinical research by lifetime suicidal ideation and behavior ascertained by the electronic Columbia-Suicide Severity Rating Scale. J Clin Psychiatry. 2013;74(9):887–93.
8. Coric V, Stock EG, Pultz J, et al. Sheehan Suicidality Tracking Scale (S–STS): Preliminary results from a multicenter clinical trial in generalized anxiety disorder. Psychiatry (Edgmont). 2009;6(1):26–31.
9. Lindenmayer JP, Czobor P, Alphs L, et al, InterSePT Study Group. The InterSePT Scale for Suicidal Thinking reliability and validity. Schizophr Res. 2003; 63:161–70.
10. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22–33.
11. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960 ;23:56–62.
12. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9.
13. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
14. Rush AJ, Giles DE, Schlesser MA, et al. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 1986;18(1):65–87.
15. Poznanski EO, Grossman JA, Buchsbaum Y, et al. Preliminary studies of the reliability and validity of the Children’s Depression Rating Scale. J Am Acad Child Psychiatry. 1984;23(2):191–7.
16. Columbia University Medical Center. Center for Suicide Assessment. Columbia-Suicide Severity Rating Scale (C-SSRS). http://www.cssrs.columbia.edu/scales_cssrs.html. Accessed October 8, 2014.
17. Reynolds WM. Psychometric characteristics of the Adult Suicidal Ideation Questionnaire in college students. J Pers Assess. 1991;56(2):289–307.
18. Gassman-Mayer C, Jiang K, McSorley P, et al. Clinical and statistical assessment of suicidal ideation and behavior in pharmaceutical trials. Clin Pharmacol Ther. 2011;90(4):554–560.