by Ashutosh Kumar, MD; Vikas Pareek, PhD; Muneeb A. Faiq, PhD; Sanjib K. Ghosh, MD; and Chiman Kumari, MD
Drs. Kumar and Ghosh are with the Department of Anatomy at the All India Institute of Medical Sciences (AIIMS) in Patna, India. Dr. Pareek is with the Computational Neuroscience and Neuroimaging Division at National Brain Research Centre (NBRC) in Manesar, Haryana, India. Dr. Faiq is with the Neuroimaging and Visual Science Laboratory at Langone Medical Centre, New York University School of Medicine in New York, New York. Dr. Kumari is with the Department of Anatomy at Postgraduate Institute of Medical Education and Research (PGIMER) in Chandigarh, India. Drs. Kumar and Faiq are with All India Institute of Medical Sciences (AIIMS) in New Delhi, India. Drs. Kumar, Pareek, Faiq, Ghosh, and Kumari are with the Etiologically Elusive Disorders Research Network (EEDRN) in New Delhi, India.
Funding: No funding was provided.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Neurogenesis in adult humans remains a controversial area of research among neuroscientists. Methodological challenges have hampered investigators from conducting high-quality, in-vivo studies that can help elucidate the presence and/or activity of neurogenesis in human brains. Additionally, the studies that have been done in humans report conflicting results, further adding to the ambiguity surrounding the concept of adult neurogenesis in humans. In this review article, the authors seek to help clarify the concept of adult neurogenesis by providing an overview of the basic concept, as we currently understand it, including its historical birth and evolution. The authors also review and discuss current key studies (pro and con) on adult neurogenesis in humans and animals, as well as research challenges with potential solutions. Finally, the authors discuss the clinical implications of adult neurogenesis in humans, based on what we know so far, including its potential use as a drug target in the development of pharmacological treatments for various neuropsychiatric disorders.
Keywords: Adult human neurogenesis, aging, hippocampus, olfactory bulb, neuropsychiatric disorders, neurorestoration
Innov Clin Neurosci. 2019;16(5–6):30–37
The concept of neurogenesis in adult humans is a controversial topic among researchers in the field of neuroscience. While some researchers report that a sharp drop in neurogenesis occurs as the human brain ages,1 other researchers report that neurogenesis in the dentate gyrus (DG) of the hippocampus of human brains persists into old age.2 A clearer understanding of the evidence surrounding the concept of adult human neurogenesis is important because its presence or absence can affect the foundations on which our concepts of the mechanisms of learning and memory are built, particularly with reference to aging, and the pathogenesis and management of many neuropsychiatric disorders.3–5 If neurogenesis in the adult hippocampus is not present, then other concepts of neuroplasticity, such as changes in synaptic transmission or remodeling of existing neurons, might move to the forefront of theory with respect to brain activity and dysfunction.6 In this review, we describe the basic concept, as well as provide a historical overview, of neurogenesis. We also critically analyze the current state of research on neurogenesis in adult humans and evaluate how the concept of neurogenesis has impacted current treatment of neuropsychiatric disorders.
Basic Concepts
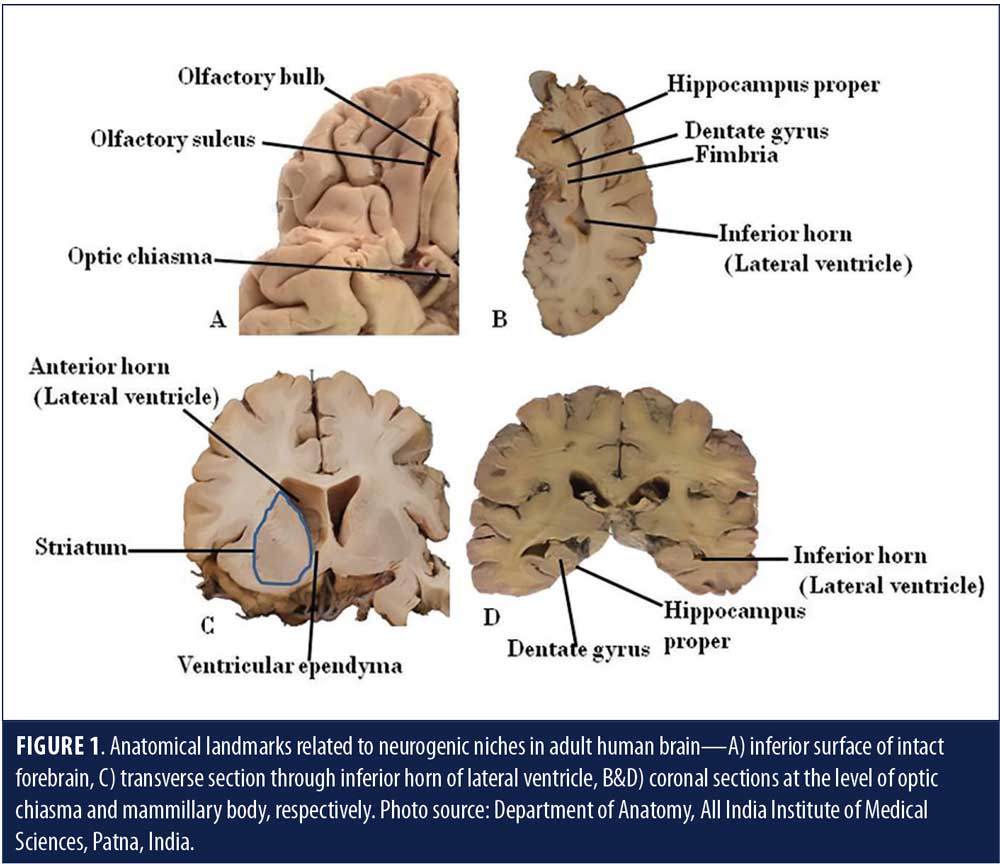
Neurogenesis is the formation of neurons de novo—the hallmark of a developing brain. In an adult animal brain, neurogenesis is said to occur in the lateral subventricular zone (SVZ) and the DG of the hippocampus (Figure 1).7
Direct corroboration of adult neurogenesis has been reported in rats, mice, song birds, and nonhuman primates.8 Indirect but considerable evidence of neurogenesis in adult humans also exists.7 In many animals, especially those bearing a strong olfactory function, such as rodents, significant adult neurogenesis in the SVZ has been reported.9 These newly formed neurons in animals migrate to forebrain regions, more particularly to the olfactory bulb, and integrate into the olfactory neural circuitry (Figure 2, Figures 3A and B).9–11
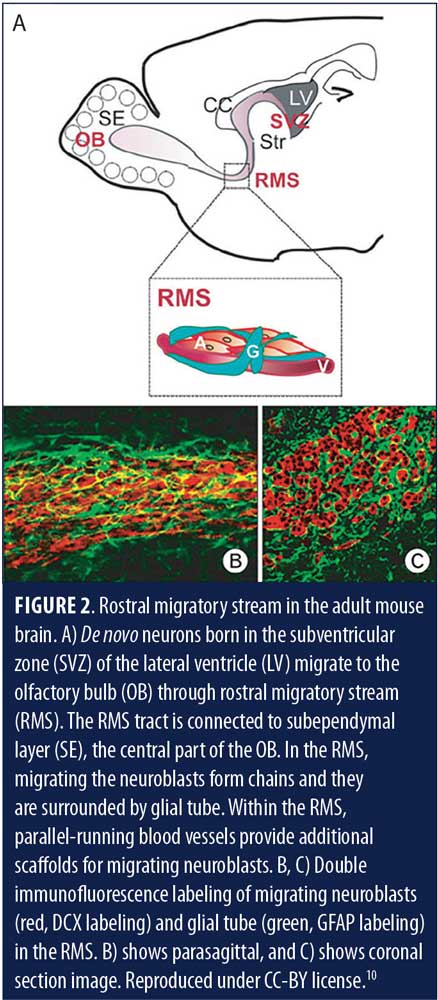
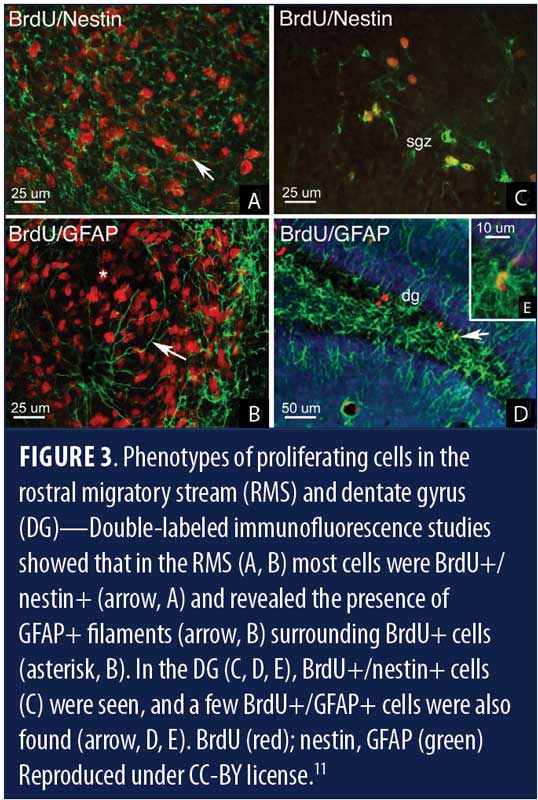
Subventricular neurogenesis is rudimentary in humans and is thought to contribute to olfactory neural circuitry and olfaction, though evidence is not explicit.12 Neurogenesis in the adult human DG has been postulated to play a role in memory and learning systems, as well as in protecting the brain from stress-induced attrition.12
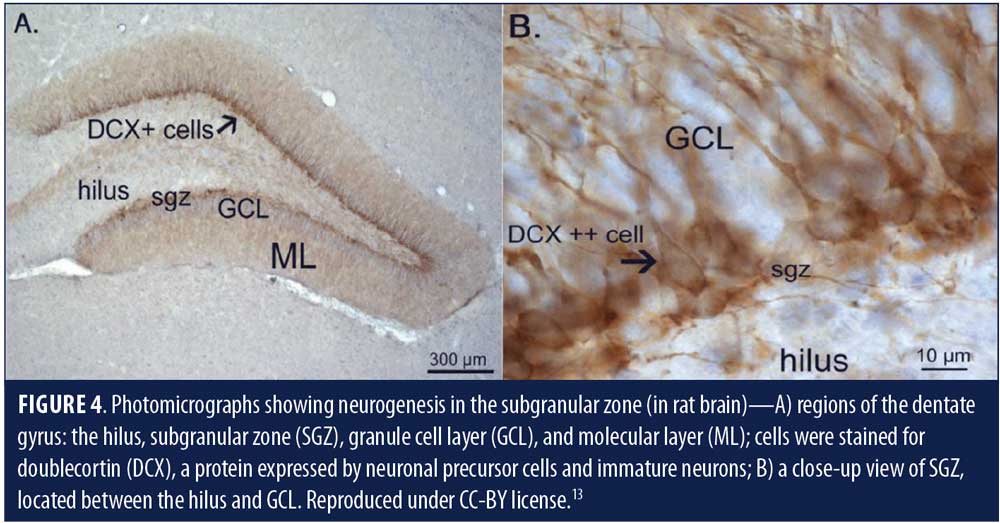
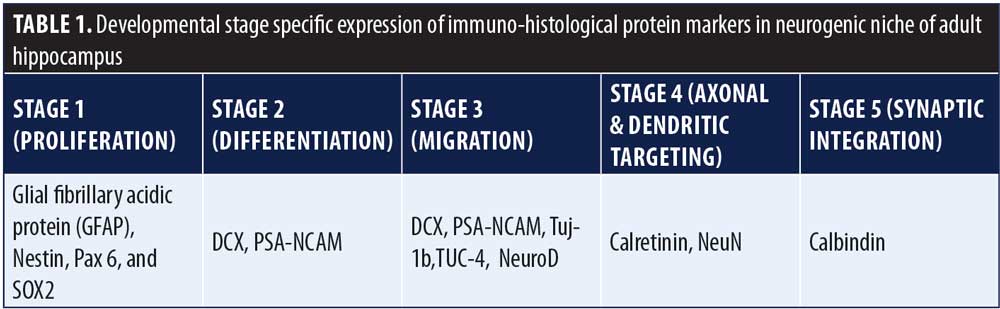
It has been proposed that human neurogenesis takes place in subgranular zone (SGZ) of the DG closer to its hilum, which maintains a neurogenic stem cell (NSC) niche (Figures 3c, d & e, Figure 4).11,13 Some researchers theorize that the SGZ is a conducive environment for the proliferation of NSCs into granule cells, from which they migrate to the granule cell layer.14 De novo adult granule cells pass through multiple developmental stages (Stages 1–5) before they can integrate into the hippocampal circuitry. These developmental stages are characterized by expression of specific protein markers, which, when observed via immunostain, reveal lineage-specific cells in the neurogenic niche (Table 1).14 Stage 1 (proliferation) is represented by NSCs, or Type 1 radial glia-like cells (RGL), marked by the expressions of glial fibrillary acidic protein (GFAP), Nestin, and SOX2 or other stem cell markers. RGLs give rise to Stage 2 (differentiation) intermediate progenitor cells (IPCs, Type 2 cells) with transient amplifying characteristics, still dividing and showing the expression of either doublecortin (DCX) or polysialylated neural cell adhesion molecule (PSA-NCAM). IPCs can give rise to Stage 3 (migration) neuronal lineage committed cells or neuroblasts (Type 3), which might show expression of both DCX and PSA-NCAM, as well as other markers of immature neurons, such as Tuj-1b and TUC-4 or NeuroD; and subsequently differentiate into Stage 4 (axonal and dendritic targeting) mature DG neurons expressing calretinin (a calcium binding protein) and NeuN (neuron-specific nuclear protein, a post-mitotic neuronal marker). These newly formed mature granule cells further integrate into the hippocampal circuitry (Stage 5 or synaptic integration), showing expression of calbindin, a calcium binding protein and a marker of synaptic integration.14 The integrated neurons can now actively influence the hippocampal functions, including learning, memory, and spatiomotor performances. The addition of new neurons is thought to provide a neural substrate to accommodate newly gained experiences, protection from attrition, resilience to stress and anxiety,3,14 and, presumably, prevent neurodegeneration.
Imayoshi et al15 studied the effects of genetic ablations of newly formed neurons in the brains of adult mice and suggested that continuous neurogenesis is required for the modulation and refinement of the existing neuronal circuits in the DG and normal behavior involved in hippocampal-dependent memory.
Enhanced hippocampal neurogenesis following exercise and schedules of environmental enrichment (i.e., sufficient availability of food, physical activity, and mating) has also been reported in animal models.16,17 Both acute and chronic stress are known to decrease hippocampal neurogenesis.18 Though the molecular mechanisms of neurogenesis in the adult brain are not fully understood, some researchers have suggested that corticosteroids might play a role in reducing hippocampal neurogenesis, and that trophic factors, including brain-derived neurotrophic factor (BDNF), fibroblast growth factor (FGF), and epidermal growth factor (EGF), and the neurotransmitter serotonin play roles in its enhancement.19–21 Stress causes an increase in secretion of glucocorticoids and decrease in that of trophic factors. Increased secretion of glucorticoids is mediated by hypothalamic-pituitary-adrenal (HPA) axis,19 and it will cause dicrease in neurogenesis. Conversely, environmental enrichment causes increase in secretion of trophic factors which in turn promote neurogenesis.17 Specific epigenetic mechanisms have also been reported to play a role in mediating positive and negative influence of environmental factors on baseline neurogenesis in animal studies.22
Adult neurogenesis in animals, including mammals, has been studied in postmortem specimens from neurogenic niches using various methods like deoxyribonucleic acid (DNA) labeling, carbon dating of the genomic DNA, immunostaining of developmental stage-specific protein markers, tracing of viral vector tagged with fluorescent reporters, introducing a viral toxin using Cre/lox system, and genetic ablation. This knocks out a neurotransmitter receptor gene (e.g., glutamate receptor), which will inhibit normal functioning of young neurons by using an inducible Cre gene or transgenic models.3,23 Behavioral effect of inhibiting neurogenesis can be studied in living animals by producing targeted, controlled lesions of neurogenic niches using drugs, viral toxins, genetic ablation, or precise irradiation.24 Studying adult neurogenesis in humans, on the other hand, has severe limitations due to a lack of a method for marking the neurogenic niches before death.
Historical Perspective
Since the birth of the “neuron doctrine” in 1894, a vital precept of contemporary neuroscience theorizing that the nervous system is made up of distinct individual cells, the general consensus for many years was that neurons could not regenerate in the adult brain.25 Santiago Ramón y Cajal, in 1913, wrote “In the adult… the nerve paths are something fixed, ended, and immutable. Everything may die, nothing may be regenerated.”26
The first evidence of adult brain neurogenesis came from Joseph Altman in the 1960s from his studies in rats. However, Altman’s experimental evidence in rat was not robust enough. At that time, adult neurogenesis in either the animal or human brain was not known at all, and any such concept was considered improbable, so his evidence was ignored by the scientific community.27 In the 1980s, Fernando Nottebohm of Rockefeller University published the first clear evidence of adult neurogenesis in song birds.28 Over time, more studies were conducted in animal models, particularly rodents, and the results were supportive of continued neurogenesis.7 One study reported that de novo neurons in the hippocampus of adult rats showed similar membrane properties and were functionally equivalent to mature granule cells and, hence, could integrate into existing circuitry to influence hippocampal functions.29
Kornack and Rakic,30 using BrdU (a thymidine analogue that labels DNA) and cell-specific markers, reported evidence supporting continued neurogenesis in the hippocampal region in adult macaque monkeys, though they estimated the rate of neurogenesis was 10 times less than in rodents. Another study reported finding BrdU labeled cells in associative neocortical regions in adult macaque monkeys, which the investigators suggested provided evidence for newly formed neurons;31 however, the study was criticized for not considering alternative explanations. A later study reported conflicting results after examining cell-specific markers that showed that newly formed cells in the new cortical regions in macaque monkeys were not neurons, though the authors supported that the presence of newly born neurons in the olfactory bulb and hippocampus was possible.32 On the other hand, a study in dolphins, porpoises, and whales—known for their large brains, longevity, and complex behavior similar to humans—reported that these mammals had relatively small hippocampi that lacked neurogenesis.33
The concept of adult neurogenesis in humans was first proposed in 1998 when Eriksson et al34 reported affirmative evidence of neurogenesis in the hippocampus after examining the postmortem tissue from patients with cancer who were injected with BrdU for therapeutic purposes.34 Knoth et al35 reported evidence of neurogenesis in the individuals 0 to 100 years of age after identifying protein markers for various stages of neurogenesis. Investigators used postmortem brain tissue samples and neurogenesis markers were identified using immunohistochemistry. In 2011, Sanai et al36 reported the discovery of the rostral migratory stream in the human brain, suggesting a rudimental continued olfactory neurogenesis. This was shown in postmortem tissue from the human brain. Rostral migratory stream (RMS) is said to carry precursor neuronal cells from the SVZ to olfactory neuroepithelium. RMS is, by default, active in the brains of animals and humans of fetal age, and as well as in many adult animals, but its presence in the adult human brain has been controversial. Another study reported observing neurogenesis in postmortem samples of human striatum, including migration of newly formed interneurons to the neocortex measuring the concentration of the nuclear bomb test derived radioactive carbon (C14) in genomic DNA,37 though no additional studies have been conducted that lend support to these results. C14 present in the environment gets integrated into the genomic DNA of the dividing cells in the body. In individuals born after 1955, around the time of the nuclear bomb tests, individuals started to show a higher concentration of C14 in their genomic DNA reflecting the higher environmental levels post tests. At any time, newly formed cells of the body will reflect the environmental concentration of C14, which can be accurately estimated. This fact has been exploited by the investigators of this study for the retrospective birth dating of the neuronal cells in the human brain to prove adult neurogenesis. The brain tissue samples for this study were collected from the postmortem human individuals admitted for the autopsy and tissue banks.
In 2013, Jonas Friesen’s lab, using carbon dating methods, reported that up to 700 neurons are added each day in human DG.38 In the following years, several additional studies supported this,8 but these studies were criticized by experts in the field who opined that BrdU can label dying cells, not dividing ones, thereby generating false signals of neurogenesis, whereas protein markers can accidentally label different types of brain cells as glia, not of neurons.1 They also postulated that the study of C14 might provide inaccurate numbers and is prone to contamination.1
Neurogenesis Research in Living Humans: Current State, Challenges, and Possible Solutions
Current state of research. The most robust study to date disputing the concept of adult human hippocampal neurogenesis was done by Sorrells et al.1 These investigators collected postmortem and postoperative hippocampal tissue (epilepsy cases) from humans. The subjects ranged in age from fetuses at 14 gestational weeks to adults 77 years old. The researchers stained the samples with the fluorescent marker antibodies Ki-67+, SOX1+, and SOX2+ to identify neural progenitor cells, and DCX+PSA-NCAM+ to identify young neurons. With electron microscopy, they also looked for the characteristic long, slender, simple shapes of young neurons. Their results showed that young neurons decreased in density from roughly 1,618 cells/mm2 at birth to 12 cells/mm2 at Age 7 to two cells/mm2 by Age 13, and almost no young neurons in the samples in individuals over the age of 18.1 A strength of the study was that they used more than one method to validate their findings, (e.g., TEM profiling of neuron lineage cells, in-situ hybridization for selected neurogenesis markers, a corresponding study in macaque monkeys, and a comparison of the gene expression of signature neurogenesis markers in developing human and monkey hippocampus in a unique mosaic).1
Prior to Sorrells et al, another study by Dennis et al39 using similar immunohistological methods also failed to demonstrate any continued neurogenesis in the adult human. The authors studied 23 individuals aged 0.2 to 59 years and observed a marked decline in neurogenesis in SVZ and SGZ in early childhood and then after three years of age. They noted that microglia were the only proliferating cells found in both niches and adjacent parenchyma.39
The most robust study that supports the validity of the concept of adult human hippocampal neurogenesis was done by Boldrini et al,2 who used similar immunohistological methods to the Sorrells study. Here investigators autopsied hippocampi (dentate gyri) from humans aged 14 to 79 years, and found that the total numbers of intermediate neural progenitors and immature neurons (that were in thousands), mature granule neurons, and glia (in its individual parts) didn’t show any significant differences between the age groups.
The evidence presented by Boldrini et al2 was considered resilient and convincing because the authors ensured that the samples were taken from healthy individuals using more biological parameters, such as angiogenesis and change in volume of DG, for a suitable comparison. They also employed unbiased stereology, which is the gold standard for counting number of neurons.2
The contrary results presented by the Sorrels study and Boldrini study highlight the existing ambiguity regarding the concept of neurogenesis in adult humans. Both studies employed reasonably similar immunohistological methods and included many of the same neurogenesis markers, yet contrasting results were observed. The study by Boldrini et al examined samples from humans aged 14 to 79 years, finding more than a thousand cells in each part of the DG, which was in stark contrast to what was observed by Sorrells et al, who found very few cells in the neurogenic niche of subjects in the same age range. Even if we consider that some discrepancies in numbers might arise due to the difference in counting methods (or subjective reasons), such marked and obvious disparity is perplexing. Sorrels et al justified their findings, noting the limitation that relying solely on the presence of markers might cause glial lineage cells to be identified as neuronal lineage cells. However, the authors stated that they used additional methods for confirmation, including transmission electron microscopy (TEM)-immunogold and in-situ hybridization. The relative paucity of any type of progenitor or immature cells, including glia in the neurogenic niche of DG in their study, remains unexplained.
Kempermann et al7 expressed skepticism regarding the negative findings of Sorrells et al, naming the postmortem interval, the lack of known status regarding neuropsychiatric disease or chronic ailment, and using patients with epilepsy as key factors of concern.7 Kempermann et al argued that, in severe epilepsy, destruction of the neurogenic niche is an explicit possibility, and that, in some cases, epilepsy could be the reason for the lack of neurogenesis.7 Additionally, Kempermann et al also criticized the use of 10% formalin in some of the samples due its potential to mask the expression of proteins.7 However, Sorrells et al clearly mentioned that they performed appropriate antigen retrieval for the selected sections.1 Kempermann et al7 suggested that dependence on the protein markers to denote neurogenesis could be an erroneous approach and that some of the markers, such as DCX, are known for fast degradation. Additionally, the presence of DCX-negative immature neurons and high inter-individual variation in expression of DCX in humans, which was also reflected in the data presented by Boldrini et al, are not unusual.7 The use of fluorescence markers also was presented as a caveat by Kempermann et al7 because it is prone to fade away and might give rise to false negative impression.
More recently, other evidence supporting hippocampal neurogenesis in adult humans has emerged. Moreno-Jiménez et al,40 using tightly controlled conditions and state-of-the-art tissue processing methods, identified thousands of immature neurons exhibiting variable degrees of maturation along the differentiation stages of the DG in neurologically healthy humans up to the ninth decade of life. These authors also reported that continued neurogenesis was reduced in the DG of patients with Alzheimer’s disease. Tobin et al41 reported observing persistent hippocampal neurogenesis in adult humans through the 10th decade of life, including patients with mild cognitive impairments and Alzheimer’s disease, though not as significant as those with no cognitive impairments.41
Our own recently published research42 used bioinformatics methods to analyze the differential expression of neurogenesis signature markers as per maturation stages in developmental transcriptome (prenatal to adult ages) in human hippocampi. Our results fall somewhere in between the results reported in other studies,1,2,39 in that we found persistent but minimal hippocampal neurogenesis in adult humans. Our data also suggest that a significant number of newborn hippocampal cells could be glial cells and not neurons, which is partially in line with results of other studies that dispute continued hippocampal neurogenesis in adult humans.1,39
Research challenges and possible solutions. Due to the rare availability of optimum human brain tissue and limitations of study methods that can be used in living humans, designing a robust study on neurogenesis that can exclude the many confounding factors seen in previous studies is a challenge. Possibly integrating the methods and approaches of both the Sorrells and Boldrini studies to a single design might yield results with an additional level of coherence, though it might also give rise to additional questions. A robust proposition would be to conduct a series of collaborative experiments by these two groups with specific, targeted objectives. Also, the use of bioinformatics methods to analyze the differential expression of neurogenesis signature markers, as per maturation stages in developmental transcriptome (prenatal to adult ages) in human hippocampus, might contribute additional clarity.43 This has been partly tried by Sorrells et al when they compared the developmental transcriptome of human hippocampus with that of macaque monkeys and found them comparable in expression of marker proteins.1
A lack of noninvasive and safe investigatory methods has hampered neurogenesis-related research in living humans to a considerable extent. Relatively safe neuroimaging approaches, which can detect growth of newly formed cells in and around neurogenic niches and their integration in the existing neural circuitry with high specificity, might be a solution.44 The use of novel techniques in stem cell biology—such as induced pluripotent cells, which is to generate neural stem cells from a patient’s own cells; and developing brain organoids from the derived neural stem cells, which facilitate study of the three-dimensional growth of the cells—might be an additional noninvasive approach to study disease-specific effects on adult neurogenesis in humans.45
Clinical Implications
Though human studies on adult neurogenesis are inadequate to date, animal model studies suggest neurogenesis potentially plays a significant role in influencing the pathogenesis of many neuropsychiatric disorders and age-related cognitive dysfunction.4,46 Animal studies have shown correlated vicissitudes in adult hippocampal neurogenesis with aging, epilepsy, stroke, neurodegenerative disorders, and psychiatric disorders.47–50 Stress and depression were also found to be associated with a decrease in neurogenesis.51 Some studies have shown that the decrease in neurogenesis might be reversed by antidepressants, antipsychotics, and/or increasing physical exercise.17,52,53
Despite optimism regarding the development of potential neurogenesis-based therapeutic approaches for neuropsychiatric disorders, available empirical evidence has not been encouraging. While evidence of a contributory role of neurogenesis in the pathogenesis and/or remission of many neuropsychiatric disorders has been bolstered by certain animal studies,4,46 the existing literature does not support modulating neurogenesis as a therapeutic strategy in most of the neuropsychiatric disorders, including depression and schizophrenia,4,46,54 and finding suitable drug targets remains a challenge.
Among the psychiatric disorders, depression has been most strongly linked with discontinuation of hippocampal neurogenesis. Early, indirect implications for a ceasing of neurogenesis as an etiogenic factor for developing depression have been observed in patients with cancer in whom prolonged treatment with anticancer drugs, which can kill newly formed cells, induced depressive symptoms.55 However, such arguments fail to account for other possible factors contributing to the depression in these patients.
Consecutive animal model studies have indicated the potential of neurogenesis-based targets in drug development for depression due to the implied role that neurogenesis plays in the mechanisms of actions of many antidepressant drugs.5,56,57 The selective serotonin reuptake inhibitor (SSRI) antidepressant, fluoxetine, for example, was found to induce a significant increase in the number of progenitor cells when used chronically in mice, which consequently led to the formation of new neurons in the hippocampus.58 Evidence from clinical trials, however, is not yet sufficient to form a definitive conclusion on this in a categorical manner.
A neurogenic drug, NSI-189 phosphate, was found to reduce severity of the symptoms in patients with major depressive disorder (MDD) compared to placebo, but the robustness of the results was limited by small sample size and skewed test-control distribution of the study,59 thereby necessitating further studies to draw any meaningful conclusions.
The hippocampus is known to influence the HPA axis-mediated stress response.60 Newly formed hippocampal neurons express receptors for the glucocorticoids/corticosteroids (end hormones of the HPA axis),61 and a dysregulation of hippocampus-HPA axis-mediated stress response has been proposed as a basis for the glucocorticoid/corticosteroid theory of depression.62,63 An abrupt discontinuation of neurogenesis in the hippocampus might potentially disrupt the HPA axis and alter the stress response.64 Plausibly, HPA axis hormones have been considered eligible drug targets in depression.65 As a proof of concept, corticotrophin releasing factor (CRF) inhibitors, which are released from the hypothalamus and trigger HPA axis activity, showed promise in animal model studies, but not in clinical trials.66
Metformin—a United States Food and Drug Administration (FDA)-approved drug for the treatment of Type 2 diabetes—was reported to induce neurogenesis in a rat model and in human neuronal cell cultures, but no clinical trials have been conducted to support these results.67 Prolonged treatment with this drug in humans with diabetes, however, was found to have an antidepressant effect and appeared to protect patients from cognitive decline.67,68
Certain studies support the hypothesis of dysregulation of continued neurogenesis in the case of the pathogenesis of psychotic disorders, especially schizophrenia.4,69 Reduced proliferation and abnormal precursor cells were observed and correlated with cognitive deficiencies in humans.4,69 Furthermore, there are indications that abnormal dendritic branching and synaptic connections of newly formed cells might be mechanistically involved in erroneous information processing, a characteristic of schizophrenia.4,69 Certain genes implicated in schizophrenia etiology, such as disrupted in schizophrenia-1 (DISC-1) and neuronal PAS domain-containing protein 3 (NPAS3), showed altered neurogenesis when knocked out in mice models, though manipulation of hippocampal neurogenesis was not found to alleviate key symptoms of the disease. Additionally, though studies have demonstrated an improvement in cognition with the enhancement of neurogenesis in animal models, evidence in humans is needed. Electroconvulsive treatments (ECT) in schizophrenia have been shown to enhance hippocampal neurogenesis, but there is no clear evidence that neurogenesis is essentially involved in the mechanism of any of the antipsychotic drugs.4,54 Though the mechanism of action is not clear, neurogenesis has also been reported to have anti-addictive effects in animal model studies, suggesting its potential use as a drug target for treatment of substance use disorder.70,71
In Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (ALS), dysregulation of neurogenesis at known niches has been reported, but results have varied among the studies, and interventional benefits were not clear.46,72 Applying a case-specific approach to address the precise aspects of neurogenesis that were affected in these diseases might be useful to study.
Currently, therapeutic application of a neurogenesis-based approach in psychiatry faces a road block. A suitable therapeutic strategy exploring neurogenesis as a treatment modality has yet to emerge. Additionally, the effectiveness of isolated targeting in continued neurogenesis as a therapeutic modality has not been substantiated by any study. Arguably, all major neuropsychiatric disorders are multifactorial in origin; thus, any influence of continued neurogenesis in disease pathogenesis or remission has many variables, which might render the isolated targeting of neurogenesis as a therapeutic approach less effective. Manipulation of continued neurogenesis could potentially serve more as an adjunctive therapy rather than a primary therapy for the treatment of neuropsychiatric disorders.
Rehabilitative methods, such as exercise and environmental enrichment, have been shown to enhance adult neurogenesis in animal models and appear to be effective in alleviating depression and cognitive decline associated with animal models of psychiatric disorders.73,74 Practice of such nonpharmacological approaches might prove beneficial, but the experimental studies have been scarce in humans.
In addition to modification of neuropsychiatric diseases, continued neurogenesis in adults has been shown to have many neurophysiological implications as well. Past studies of animal models have suggested that neurogenesis-induced plasticity might boost learning and memory and might possibly play a role in stress response and emotion.75 Rodent studies have indicated disruption of contextual fear conditioning when ongoing neurogenesis is inhibited.76 In addition to the relevance in diseases, continued neurogenesis in adults, especially in the hippocampus, also suggests its potential role in normal day-to-day life. Augmentation of neurogenesis in animals has been observed to improve pattern separation, hence spatial memory performances.17 A study in adult mice suggested that the pace and extent of hippocampal neurogenesis are dependent on neural activity.77 Activity-dependent neurogenesis in the hippocampus might be instrumental in improving the memories of new life experiences and spatial learning.77 In turn, standardized learning modules can induce neurogenesis in the hippocampus.78 In this sense, regular addition of even a minimum number of new neurons might have a rejuvenating effect on the existing neural circuitry. It is possible that a minimal baseline neurogenesis can be maintained in adult humans, increasing or decreasing as needed according to the physical or mental challenges being encountered by the individual, as was observed in animal model studies.16,79 Observation, from the evolutionary point of view, when comparing studies of multiple animal species, including humans, there appears to be wide variation in the level of neurogenesis in adults. As a cardinal feature in all animal species, neurogenesis appears to decline significantly with age, with a peak value near puberty.80 Researchers have suggested that the level of neurogenesis might depend on the normal survival challenges a species generally faces during the periods of growth or thereafter. Some scholars suggest that a higher level of neurogenesis during periods of postnatal growth might be due to characteristic exploratory behavior that manifests during this period, which then stabilizes in adulthood. Theoretically, a young adult might maintain a higher level of neurogenesis, compared to an aging individual, due to the necessity of accommodating more new life experiences.49,80 If we believe this theory, a continuity of neurogenesis in adults might not have much significance except when one faces certain challenges or stressors that occur throughout life. An animal species that is better adapted to its environment and faces lesser survival challenges might have a lesser degree of continued neurogenesis in the hippocampus.49,80 This scenario, however, is counterintuitive because stress here enhances neurogenesis rather than diminish it. Continued neurogenesis in adults has been found to be a constant feature in most of the vertebrates studies to date, suggesting its evolutionary benefit to the organism and the species as a whole. Kempermann, an expert in adult neurogenesis research, proposed the hypothesis that continuity of neurogenesis in the hippocampus might have offer the evolutionary advantage of retaining an adaptable, activity-dependent neural network; in other words, the hippocampus maintains a neurogenic reserve, which allows the brain to remain flexible and plastic to facilitate learning when the individual is exposed to novelty and complexity. The activity-dependent incorporation of even a low number of neurons into the hippocampal network can lead to a functional benefit along an individual’s course of life by helping the individual to adapt to new life experiences.49
Conclusion
Though the concept of continued neurogenesis in adults has been shown to exist in animals, there is insufficient evidence to date that adequately supports its existence in adult humans. Additional studies exploring the dynamic changes in neurogenesis in the known regions of the human brain, with reference to the physiological and diseased conditions, are needed. Advancement in neuroimaging methods, complemented with biological manipulations, might help us devise in-vivo and real-time studies on neurogenesis in animal models, which might help us better explain the dynamics of neurogenesis. Future in-vivo studies in humans are needed to confirm the presence (or lack thereof) of neurogenesis in adult humans; however, methodological advancements that allow safer, deep, and unambiguous methods of investigation will be required.
Acknowledgments
We are thankful to Dr. Rizwana Qadri, Department of Laboratory Medicine, All India Institute of Medical Sciences, New Delhi for her contribution in the editing of the manuscript.
References
- Sorrells SF, Paredes MF, Cebrian-Silla A, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555(7696):377–381.
- Boldrini M, Fulmore CA, Tartt AN, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22(4):589–599.e5.
- Kempermann G, Song H, Gage FH. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol. 2015;7(9):a018812.
- Kang E, Wen Z, Song H, et al. Adult neurogenesis and psychiatric disorders. Cold Spring Harb Perspect Biol. 2016;8(9) pii: a019026.
- Apple DM, Fonseca RS, Kokovay E. The role of adult neurogenesis in psychiatric and cognitive disorders. Brain Res. 2017;1655:270–276.
- Kays JL, Hurley RA, Taber KH. The dynamic brain: neuroplasticity and mental health. J Neuropsychiatry Clin Neurosci. 2012;24(2):118–124.
- Kempermann G, Gage FH, Aigner L, et al. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23(1):25–30.
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22(3):612–613.
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22(3):629–634.
- Sun W, Kim H, Moon Y. Control of neuronal migration through rostral migration stream in mice. Anat Cell Biol. 2010;43(4):269.
- Faiz M, Acarin L, Castellano B, Gonzalez B. Proliferation dynamics of germinative zone cells in the intact and excitotoxically lesioned postnatal rat brain. BMC Neurosci. 2005;12;6(1):26.
- Bergmann O, Spalding KL, Frisén J. Adult neurogenesis in humans. Cold Spring Harb Perspect Biol. 2015;7(7):a018994.
- Oomen CA, Girardi CEN, Cahyadi R, et al. Opposite effects of early maternal deprivation on neurogenesis in male versus female rats. PLoS One. 2009;4(1):e3675.
- von Bohlen und Halbach O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007;329(3):409–420.
- Imayoshi I, Sakamoto M, Ohtsuka T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153–1161.
- Farmer J, Zhao X, van Praag H, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male sprague–dawley rats in vivo. Neuroscience. 2004;124(1):71–79.
- Sahay A, Scobie KN, Hill AS, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470.
- Aloe L, Alleva E, Fiore M. Stress and nerve growth factor: findings in animal models and humans. Pharmacol Biochem Behav. 2002;73(1):159–166.
- Snyder JS, Soumier A, Brewer M, et al. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–461.
- Bath KG, Lee FS. Neurotrophic factor control of adult SVZ neurogenesis. Dev Neurobiol. 2010;70(5):339–349.
- Alenina N, Klempin F. The role of serotonin in adult hippocampal neurogenesis. Behav Brain Res. 2015;277:49–57.
- Covic M, Karaca E, Lie DC. Epigenetic regulation of neurogenesis in the adult hippocampus. Heredity (Edinb). 2010;105(1):122–134.
- Enikolopov G, Overstreet-Wadiche L, Ge S. Viral and transgenic reporters and genetic analysis of adult neurogenesis. Cold Spring Harb Perspect Biol. 2015;7(8):a018804.
- Cameron HA, Glover LR. Adult neurogenesis: beyond learning and memory. Annu Rev Psychol. 2015;66(1):53–81.
- Palmer, C. The Neuron Doctrine, circa 1894. The Scientist. 2013. http://www.the-scientist.com/?articles.view/articleNo/37954/title/The-Neuron-Doctrine–circa-1894/. Accessed June 29, 2019.
- Ramón y Cajal S. Degeneration and Regeneration of the Nervous System [Spanish]. May RM (ed). Oxford University Press, London. 1928.
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135(3509):1127–1128.
- Nottebohm F. Neuronal replacement in adulthood. Ann N Y Acad Sci. 1985;457(1):143–161.
- van Praag H, Schinder AF, Christie BR, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034.
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96(10):5768–5773.
- Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286(5439):548–552.
- Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294(5549):2127–2130.
- Patzke N, Spocter MA, Karlsson KÆ, et al. In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis. Brain Struct Funct. 2015;220(1):361–383.
- Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317.
- Knoth R, Singec I, Ditter M, et al. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5(1):e8809.
- Sanai N, Nguyen T, Ihrie RA, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478(7369):382–386.
- Ernst A, Alkass K, Bernard S, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156(5):1072–1083.
- Spalding KL, Bergmann O, Alkass K, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227.
- Dennis CV, Suh LS, Rodriguez ML, et al. Human adult neurogenesis across the ages: an immunohistochemical study. Neuropathol Appl Neurobiol. 2016;42(7):621–638.
- Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25(4):554–560.
- Tobin MK, Musaraca K, Disouky A, et al. Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell. 2019;24(6):P974–982.E3.
- Kumar A, Pareek V, Faiq MA, et al. Transcriptomic analysis of the neurogenesis signature suggests continued but minimal neurogenesis in the adult human hippocampus. bioRxiv. 2019;1:664995. https://doi.org/10.1101/664995
- Kumar A, Pareek V, Singh HN, et al. Neurogenesis in adult human hippocampus, what signature genes expressions tell us?. Program No. 029.02. 2018 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2018. Online. https://abstractsonline.com/pp8/#!/4649/presentation/37213.
- Ho NF, Hooker JM, Sahay A, et al. In vivo imaging of adult human hippocampal neurogenesis: progress, pitfalls and promise. Mol Psychiatry. 2013;18(4):404–416.
- Wen Z, Christian KM, Song H, Ming G. Modeling psychiatric disorders with patient-derived iPSCs. Curr Opin Neurobiol. 2016;36:118–127.
- Winner B, Winkler J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2015;7(4):a021287.
- Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neurosci. 2003;9(4):261–272.
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10(9):1110–1115.
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31(4):163–169.
- Winner B, Melrose HL, Zhao C, et al. Adult neurogenesis and neurite outgrowth are impaired in LRRK2 G2019S mice. Neurobiol Dis. 2011;41(3):706–716.
- Mahar I, Bambico FR, Mechawar N, Nobrega JN. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev. 2014;38:173–192.
- Kempermann G, Fabel K, Ehninger D, et al. Why and how physical activity promotes experience-induced brain plasticity. Front Neurosci. 2010;4:189.
- Chikama K, Yamada H, Tsukamoto T, et al. Chronic atypical antipsychotics, but not haloperidol, increase neurogenesis in the hippocampus of adult mouse. Brain Res. 2017;1676:77–82.
- Yun S, Reynolds RP, Masiulis I, Eisch AJ. Re-evaluating the link between neuropsychiatric disorders and dysregulated adult neurogenesis. Nat Med. 2016;22(11):1239–1247.
- Pereira Dias G, Hollywood R, Bevilaqua MC, et al. Consequences of cancer treatments on adult hippocampal neurogenesis: implications for cognitive function and depressive symptoms. Neuro Oncol. 2014;16(4):476–492.
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–9110.
- Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809.
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103(21):8233–8238.
- Fava M, Johe K, Ereshefsky L, et al. A Phase 1B, randomized, double blind, placebo controlled, multiple-dose escalation study of NSI-189 phosphate, a neurogenic compound, in depressed patients. Mol Psychiatry. 2016;21(10):1372–1380.
- Phillips LJ, McGorry PD, Garner B, et al. Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: implications for the development of psychotic disorders. Aust N Z J Psychiatry. 2006;40(9):725–741.
- Cameron HA, Woolley CS, Gould E. Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus. Brain Res. 1993;611(2):342–346.
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477.
- Dinan TG. Glucocorticoids and the genesis of depressive illness a psychobiological model. Br J Psychiatry. 1994;164(3):365–371.
- Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr Opin Neurobiol. 2015;30:51–58.
- Maric NP, Adzic M. Pharmacological modulation of HPA axis in depression–new avenues for potential therapeutic benefits. Psychiatr Danub. 2013;25(3):299–305.
- Spierling SR, Zorrilla EP. Don’t stress about CRF: assessing the translational failures of CRF 1 antagonists. Psychopharmacol. 2017;234(9–10):1467–1481.
- Potts MB, Lim DA. An old drug for new ideas: metformin promotes adult neurogenesis and spatial memory formation. Cell Stem Cell. 2012;11(1):5–6.
- Guo M, Mi J, Jiang QM, et al. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin Exp Pharmacol Physiol. 2014;41(9):650–656.
- Allen KM, Fung SJ, Weickert CS. Cell proliferation is reduced in the hippocampus in schizophrenia. Aust N Z J Psychiatry. 2016;50(5):473–480.
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30(1):304–315.
- Mandyam CD, Koob GF. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 2012 ;35(4):250–260.
- Horgusluoglu E, Nudelman K, Nho K, Saykin AJ. Adult neurogenesis and neurodegenerative diseases: a systems biology perspective. Am J Med Genet B Neuropsychiatr Genet. 2017;174(1):93–112.
- Schoenfeld TJ, Cameron HA. Adult neurogenesis and mental illness. Neuropsychopharmacology. 2015;40(1):113–128.
- Ernst C, Olson AK, Pinel JPJ, et al. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J Psychiatry Neurosci. 2006;31(2):84–92.
- Apple DM, Fonseca RS, Kokovay E. The role of adult neurogenesis in psychiatric and cognitive disorders. Brain Res. 2017;1655:270–276.
- Shors TJ, Townsend DA, Zhao M, et al. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12(5):578–584.
- Piatti VC, Davies-Sala MG, Espósito MS, et al. The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J Neurosci. 2011;31(21):7715–7728.
- Gould E, Beylin A, Tanapat P, et al. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2(3):260–265.
- Czéh B, Müller-Keuker JIH, Rygula R, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32(7):1490–1503.
- Amrein I, Lipp H-P. Adult hippocampal neurogenesis of mammals: evolution and life history. Biol Lett. 2009;5(1):141–144.





