 by J. Cara Pendergrass, PhD; Steven D. Targum, MD; and John E. Harrison, BSc (hons), PhD, Cpsychol, CSCI
by J. Cara Pendergrass, PhD; Steven D. Targum, MD; and John E. Harrison, BSc (hons), PhD, Cpsychol, CSCI
Drs. Pendergrass and Targum are with Bracket Global and Clintara LLC, A Bracket Company, in Boston, Massachusetts. Dr. Harrison is with the Alzheimer’s Center and Department of Neurology, Neuroscience Campus Amsterdam, VU University Medical Center in Amsterdam, Netherlands; Institute of Psychiatry, Psychology, and Neuroscience (IoPPN), King’s College London, United Kingdom; and Metis Cognition Ltd, Park House, Kilmington Common in Wiltshire, United Kingdom.
Innov Clin Neurosci. 2017;15(1–2):36–44.
FUNDING: Funding provided by Bracket Global LLC.
DISCLOSURES: Drs. Pendergrass and Targum are employees of Bracket LLC. In the last two years, Dr. Harrison has received consultancy fees and honoraria from the following organizations: Abbvie; Access to Quality; Amgen; Anavex; AstraZeneca; Avonex; Avraham; Axon; Axovant; Biogen Idec; Boehringer Ingelheim; Bracket; Cambridge Brain Sciences; Catenion; CRF Health; DeNDRoN; Eisai; Eli Lilly; EnVivo Pharma; Enzymotec; ePharmaSolutions; Forum Pharma; Fresh Forward; GfHEu; Heptares; Janssen AI; Johnson & Johnson; Kaasa Health; Kyowa Hakko Kirin; Lundbeck; MedAvante; Merck; Mind Agilis; MyCognition; Neurim; Neurocog; Neurotrack; Novartis; Nutricia; Orion Pharma; Pfizer; Pharmanet/i3; Prana Biotech; PriceSpective; Probiodrug; Prophase; Prostrakan; Regeneron; Reviva; Roche; Sanofi; Servier; Shire; Takeda; TCG; TransTech Pharma & Velacor. He has additionally received royalties from Oxford University Press and Blackwell Publishers, holds patents with MyCognition and has share options in Neurotrack. He has also presented on behalf of Medscape & Lundbeck in CME accredited speaker bureau programs.
ABSTRACT: This brief review explores the areas of cognitive impairment that have been observed in cancer patients and survivors, the cognitive assessment tools used, and the management of the observed cognitive changes. Cognitive changes and impairment observed in patients with cancer and those in remission can be related to the direct effects of cancer itself, nonspecific factors or comorbid conditions that are independent of the actual disease, and/or the treatments or combination of treatments administered. Attention, memory, and executive functioning are the most frequently identified cognitive domains impacted by cancer. However, the prevalence and extent of impairment remains largely unknown due to marked differences in methodology, definitions of cognitive impairment, and the assessment measures used. Assessment of cognitive functioning is an important and necessary part of a comprehensive oncological care plan. Research is needed to establish a better understanding of cognitive changes and impairments associated with cancer so that optimal patient outcomes can be achieved.
KEYWORDS: Cognition, cognitive impairment, cancer, chemotherapy, treatment, neuropsychological assessment
INTRODUCTION
It is estimated that there will be approximately 70 million cancer survivors worldwide by the year 2020.[1,2] Cognitive impairment is commonly observed in patients with cancer and those in remission.[3–6] A national cross-sectional survey reported that a history of cancer was associated with a 40-percent increased likelihood of self-reported memory problems.[7] In a recent review, Janelsins et al4 noted that up to 30 percent of patients with cancer exhibit cognitive impairment prior to treatment, 75 percent might have measurable cognitive impairment during treatment, and 35 percent of cancer survivors will continue to exhibit cognitive difficulties in the months to years that follow treatment. Cognitive impairment can have a negative impact on daily functioning, quality of life, and capacity to work among patients with cancer and those in remission. Consequently, it is clear that increased attention is needed to fully understand the presence and nature of cognitive impairment in patients with cancer and those in remission.
DIRECT EFFECTS OF CANCER ON COGNITION
Cancer-related cognitive changes and impairment might be due to the cancer itself. Impairments associated with brain tumors often are specific to the lesion location, such as occipital tumors that result in visual deficits. The location and momentum of the lesion (i.e., the rate of tumor growth that can result in the destruction, crowding, displacement, and infiltration of brain tissue) influence the presence, intensity, and pattern of resulting cognitive changes in patients with brain tumors.[8] Wefel et al[9] reported that patients with high-grade gliomas often demonstrate greater cognitive impairment overall[ compared to those with low-grade gliomas, which might be attributed to greater invasion and/or increased pressure in nearby normal brain tissue. Up to 90 percent of patients with brain metastases exhibit some cognitive impairment prior to treatment, with the degree of impairment correlated with total lesion volume rather than the number of metastatic lesions.[10]
Patients with brain tumors can experience impairments in attention, memory, and executive function.[11–15] A general, more diffuse frontal-subcortical pattern of cognitive impairment often occurs in addition to the specific cognitive deficits related to specific location of the cancer in patients with brain tumors. A systematic review of 17 studies of cognitive functioning in patients with low-grade glioma reported a wide range, 19 to 83 percent, of prevalence of cognitive impairments, which was attributed to multiple differences across studies, including the characteristics of the glioma (e.g., type, location, and size), the time of measurement, the varied cognitive tests used, and the definition of cognitive impairment that was applied.[16] In a retrospective study of 68 patients with brain tumors, Lageman et al[17] found that 58.8 percent of the patients exhibited cognitive impairment as defined by standard deviations of 2 or greater below the published normative means on at least one subtest of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). In this study, 90 percent of the cognitive impairment occurred within the domains of visuoconstruction, processing speed, and verbal memory.[17] Of the domains listed by the authors, processing speed is better considered an outcome metric than a cognitive domain, as slowing is caused by deficits in attention, working memory, and executive function deficits.
Cancer-related cognitive changes and impairment also have been documented in patients with non-central nervous system (non-CNS) cancer prior to exposure to treatment interventions. Several studies have identified cognitive impairment in newly diagnosed patients with cancer across several cognitive domains, including verbal memory, language, visual spatial skills, executive function, and psychomotor function.[18–23] Approximately 11 to 35 percent of women with breast cancer are reported to have cognitive impairment prior to treatment, with specific difficulties on measures of learning and memory.[24–26] Patients with newly diagnosed large or locally advanced breast cancer demonstrated a range of significant impairments in attention, immediate and delayed word recall, and word and picture recognition on a computerized assessment (Cognitive Drug Research [CDR] system) prior to initiating primary chemotherapy, with notable large impairments in the speed of picture recognition (30%) and delayed word recall (25%).[24]
EFFECTS OF COMORBID FACTORS ON COGNITION
Several comorbid factors can impact cognitive functioning in patients with cancer and those in remission. Depression, anxiety, and fatigue can adversely affect cognitive functioning in healthy and ill patient populations. These symptoms are common and are particularly important comorbid conditions in patients with cancer. Berman et al[27] found that pretreatment worry was associated with subjective and objective measures of cognitive impairment, and alterations in brain function, as measured by functional magnetic resonance imaging (fMRI), were also observed in patients with breast cancer about to receive either chemotherapy or radiation treatment. Other studies have found a relationship between reported mood symptoms and subjective reports of cognitive impairment in patients with cancer.[20,28,29] One study of 40 patients with breast cancer found that anxiety, depression, and poor quality of life correlated with self-reported cognitive concerns but not with impaired cognitive performance.[30] Subjective cognitive complaints were associated with measures of fatigue and distress but not with objective performance on cognitive testing in a study of 53 survivors of breast cancer at least two years out from diagnosis.[31] In one study examining the cognitive functioning of lymphoma survivors using the CNS Vital Signs (Chapel Hill, North Carolina) computerized assessment, fatigue and anxiety were related to subjective reports of cognitive difficulties, whereas pain was associated with performance deficits on objective cognitive measures.[32] Fatigue can significantly impact daily functioning and quality of life in patients with cancer.[33,34] This is perhaps unsurprising given the importance of cognition in the execution and initiation of many activities of daily living.
Treatment expectations might also influence cognitive function in some patients with cancer. One recent study found that patients treated with chemotherapy who were informed of the possible cognitive side effects performed worse on cognitive testing and were more likely to report problems compared to comparable patients who were not informed about the possible cognitive side effects.[35]
DIRECT EFFECTS OF CANCER TREATMENTS ON COGNITION
It is well established that cancer chemotherapy can induce cognitive impairment.[6] It has been estimated that 13 to 70 percent of patients receiving cancer chemotherapy have measurable cognitive impairment.[3,20,25,26,36] As noted earlier, the wide range of cognitive impairment that has been reported relates to the diversity of definitions and tests that are used. The observed cognitive impairment might or might not resolve for some patients following treatment, and consequently, some patients might experience persistent, long-term cognitive problems.[5]
Several recent meta-analyses examining cognitive impairment following oncological treatments have shown that patients with cancer and those in remission have impairment in the domains of memory, attention, executive function, “processing speed,” visual and verbal memory, and language relative to people without cancer.[2,11,26,37–40] One study observed moderate-to-severe cognitive impairment in 16 percent of patients receiving adjuvant or neoadjuvant chemotherapy for breast cancer compared to only four percent of typical controls.[41] Cognitive function was examined in 196 long-term survivors of breast cancer treated with cyclophosphamide, methotrexate, and fluorouracil (CMF) who were, on average, 21 years out from diagnosis. The researchers found that the chemotherapy group performed significantly worse on several cognitive tests, including immediate and delayed verbal memory, executive functioning, and psychomotor speed compared to 1,509 control patients with no history of cancer.[29] A separate study of 189 survivors of breast cancer found that memory and executive function complaints were present in approximately 20 percent of the cohort and showed a statistically significant association with results of domain-specific cognitive tests.[42] It is noteworthy that age was not a moderating factor of cognitive impairment in the reported meta-analyses.[2,39,40] This observation suggests that aged individuals are not more susceptible to the negative impacts on cognition of cancer treatments.
PUTATIVE MECHANISMS AFFECTING COGNITION DURING CHEMOTHERAPY
Elevated levels of cytokines, deoxyribonucleic acid (DNA) damage, or white matter damage might contribute to cognitive deficits,[43–47] and the risk of developing chemotherapy-associated neurotoxicity might be related to exposure to higher doses,[48] additive and synergistic effects of multi-agent chemotherapy,[8] and administration of chemotherapy via intra-arterial and/or intrathecal methods.[49] Several articles by Ahles and colleagues have been published discussing different proposed mechanisms for cancer-associated cognitive impairments, including recent research exploring the relationship between cognitive changes in cancer patients with the aging process and genetic factors.[43,44,50]
Cytokines have important roles in normal CNS function, including the modulation of neuronal and glial cell functioning and neural repair and the metabolism of dopamine and serotonin.[43] Cytokine neurotoxicity has been found during early stages of cancer and is triggered by cancer chemotherapy as well, possibly contributing to the emerging cognitive impairments.[43] Increased cytokine levels (e.g., interleukin [IL]6, IL8, and IL10) have been associated with chemotherapy drugs such as paclitaxel and docetaxel.[51–54] Longitudinal studies on interferon-alpha and IL-2 treatments in cancer populations have shown cognitive impairments in information processing speed, executive function, spatial ability, and reaction time that were independent of depressive symptoms.[55–57]
The chemotherapeutic agents used to treat cancer-induced tumor cell apoptosis through DNA damage[43,58,59] can also impact normal cells. Cytokine neurotoxicity might be triggered by the DNA damage, setting up a cycle of increasing DNA damage and increased cytokine activity.[60] Oxidative DNA damage has been identified in peripheral blood lymphocytes following chemotherapy for breast cancer,[61,62] and an increased number of point mutations in mitochondrial DNA has been detected following cancer chemotherapy with or without radiation.[63] Chemotherapy also has been associated with increased oxidative stress, and DNA damage increased levels of nonprotein-bound iron,[64] increased free radicals,[65] and reduced antioxidant activity.[65–67] Oxidative DNA damage has also been found in patients with breast cancer prior to receiving chemotherapy,[61,62] suggesting that deficits in DNA-repair mechanisms are associated with an increased risk of cancer[68,69] and might also contribute to the cognitive symptoms noted prior to treatment.
Both brain atrophy and white matter pathology have been observed after chemotherapy in patients with breast cancer. Longitudinal studies have shown reductions in gray matter, primarily in bilateral frontal regions and the hippocampus.[70] Only partial gray matter recovery has been found within one-year post-chemotherapy compared to cancer patients who did not receive chemotherapy and compared to healthy controls.[70] Changes in overall gray matter volume equivalent to four additional years of aging have also been observed in survivors of breast cancer after more than 20 years.[29] Evidence of reduced white matter integrity, believed to reflect axonal degeneration and demyelination, has been reported using diffusion tensor imaging.[8] Specifically, decreased frontal, parietal, and occipital white matter integrity have been found in patients treated with chemotherapy compared to cancer patients who had not received chemotherapy and compared to healthy controls.[45] Several imaging studies suggest that the pattern of treatment-related cognitive impairment seen in patients with cancer and those in remission is related to functional and structural changes in the brain.[44–47] For instance, hypoactivation in the prefrontal cortex has been observed during memory encoding and executive function tasks in patients with breast cancer during chemotherapy.[71] Furthermore, a prospective, longitudinal treatment study on patients with breast cancer tracked alterations in frontal activation during a working memory task. The authors reported evidence of hyperactivation at baseline, a relative decrease at one month post-completion of chemotherapy, and a return to hyperactivation after one year, which was interpreted as reflecting a compensatory recruitment of neural circuitry.[72] Altered resting-state functional brain network activity characterized by disrupted frontal, striatal, and temporal areas has been observed in patients with breast cancer treated with chemotherapy compared to typical controls.[73,74] Patterns of disrupted default mode network (DMN) that were distinct from DMN connectivity patterns seen in patients with breast cancer who were not treated with chemotherapy or in typical controls have been reported in imaging studies of patients with breast cancer treated with chemotherapy.[75] Kessler et al[75] reported that the alterations in DMN connectivity were associated with patient-reported disturbances in memory.
EFFECTS OF ADJUNCTIVE TREATMENTS ON COGNITION
Adjunctive treatments, such as endocrine therapy, can induce cognitive impairment in patients with cancer.[2,36,44,76,77] A longitudinal study of cognitive performance in breast cancer found that use of adjuvant endocrine therapy was associated with slowed processing speed and verbal memory.[37] Patients with breast cancer who received combined chemotherapy and endocrine therapies have been reported to exhibit poorer scores on a working memory task compared to patients receiving either chemotherapy or endocrine therapy alone.[78] Furthermore, a longitudinal study reported that treatment-induced menopause was associated with cognitive impairment following chemotherapy in patients with early stage breast cancer.[79]
EFFECTS OF RADIATION TREATMENTS ON COGNITION
Patients receiving brain radiation treatments often experience radiation-induced fatigue and headache, in addition to possible cognitive impairment. Whole brain radiotherapy (WBRT) has been shown to worsen fatigue in patients with cancer.[80,81] The European Organization for Research and Treatment of Cancer (EORTC) reported that patients who received WBRT had measurable cognitive decline that they attributed to fatigue, as well as clinically significant higher fatigue scores, compared to patients with surgical or radiosurgical management.[80] Chang et al[82] found that adding WBRT to stereotactic radiosurgery increased the risk of learning and memory impairment at four months post-treatment, compared to patients who were treated only with stereotactic radiosurgery.
Patients who have received radiotherapy are at risk of developing subacute toxicity approximately 1 to 6 months after completion of radiotherapy. This effect has been associated with impairment in processing information, attention, verbal memory, executive functioning, and fine motor dexterity.8 White matter changes have been associated with these cognitive changes, and post-radiotherapy white matter recovery might result in cognitive recovery over time.[8] Numerous risk factors for developing radiation-induced cognitive impairment include age (under 5 years or older than 60 years), greater than 2-Gy dose per fraction, higher total dose, hyperfractionated schedules, shorter overall treatment time, the presence of comorbid vascular risk factors, concomitant or subsequent treatment with chemotherapy, and a greater total volume of brain irradiated.[83,84] Long-term memory impairment has been associated with increased exposure of radiation to the bilateral hippocampi.[85] Late-delayed toxicity from radiotherapy can occur months to years after completion of treatment and can include severe, irreversible memory loss.[8,86] Consequently, monitoring of cognitive and daily functioning in patients with cancer who receive radiotherapy is necessary long-term and in the immediate post-treatment period.
ASSESSMENT OF COGNITIVE FUNCTIONING IN PATIENTS WITH CANCER AND THOSE IN REMISSION
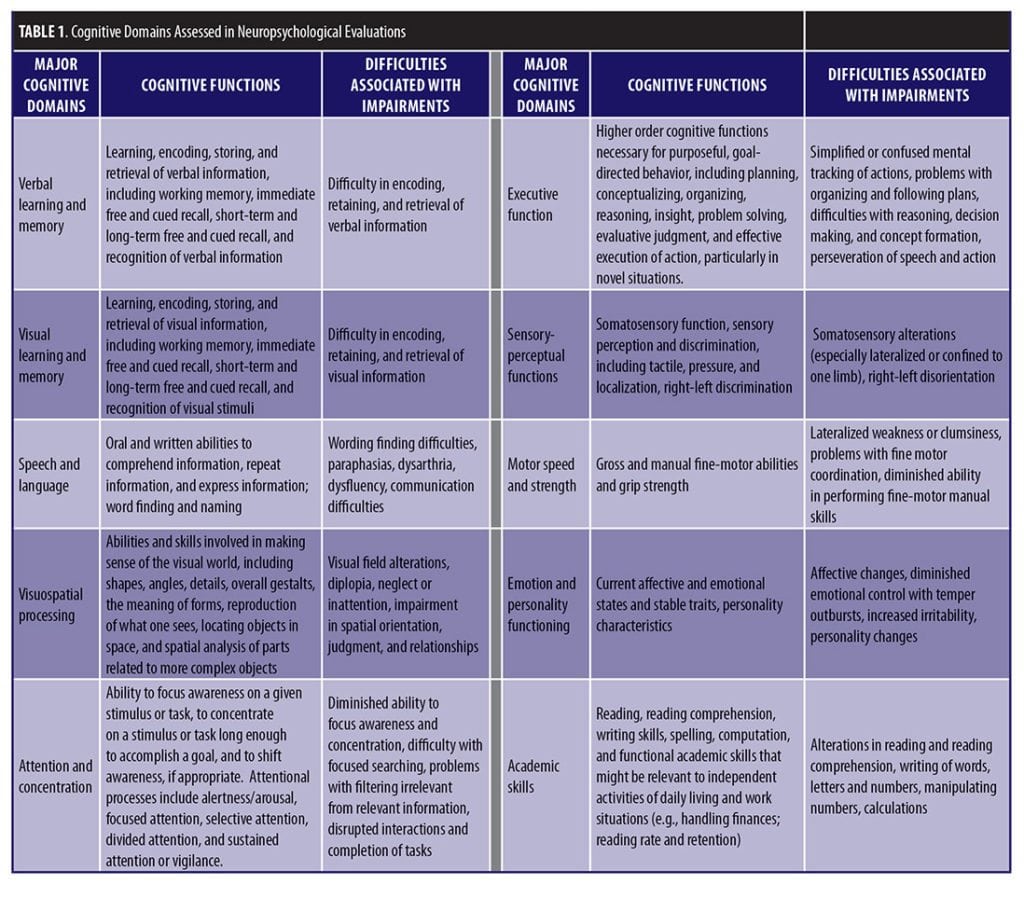
The assessment of cognitive functioning in patients with cancer and those in remission is a crucial component of a comprehensive oncological treatment plan. The delineation of cognitive changes over time is particularly helpful when clarity is needed to guide possible management and treatment intervention.[5] Evaluation of cognitive functioning in patients with cancer might also be needed for evaluation of disability benefits or work limitations. Oncology cognitive evaluations should emphasize tests that assess frontal-subcortical network functioning, including learning and memory, executive functioning, speed of processing, and speeded motor coordination. Table 1 describes the major domains that are often included in cognitive assessments. Ideally, a pretreatment, baseline evaluation followed by serial assessments to gauge the trajectory of cognitive changes and impairment over time are recommended.[5] Currently, a universally accepted, standardized set of cognitive assessment measures does not exist for the evaluation of cancer-associated cognitive changes and impairment. In fact, the literature on cancer and cancer therapy-related cognitive impairment reveals marked differences in the definitions used, the cognitive domains explored, and the types of assessment measures used to evaluate cognitive functioning.
Meyers and Brown[87] and Wefel and colleagues[88] developed their own cognitive test batteries to assess cancer patients. These assessments consisted of reliable, well-known cognitive measures: Digit Span (attention); Digit Symbol (information processing); Block Design and Similarities of the WAIS-III (visuoconstruction and conceptual formation, respectively); Trail Making Test: Trails A and B (attention and executive functioning); Hopkins Verbal Learning Test (verbal learning and memory); Grip Strength (motor functioning); Grooved Pegboard (fine motor coordination); Boston Naming Test (naming objects); Token Test (receptive language); and Controlled Word Association Test (COWA) (executive functioning spontaneous language production). However, some of these measures were not designed for repeated, serial assessment, and the lack of parallel versions for some of the measures undermines their utility in longitudinal studies.
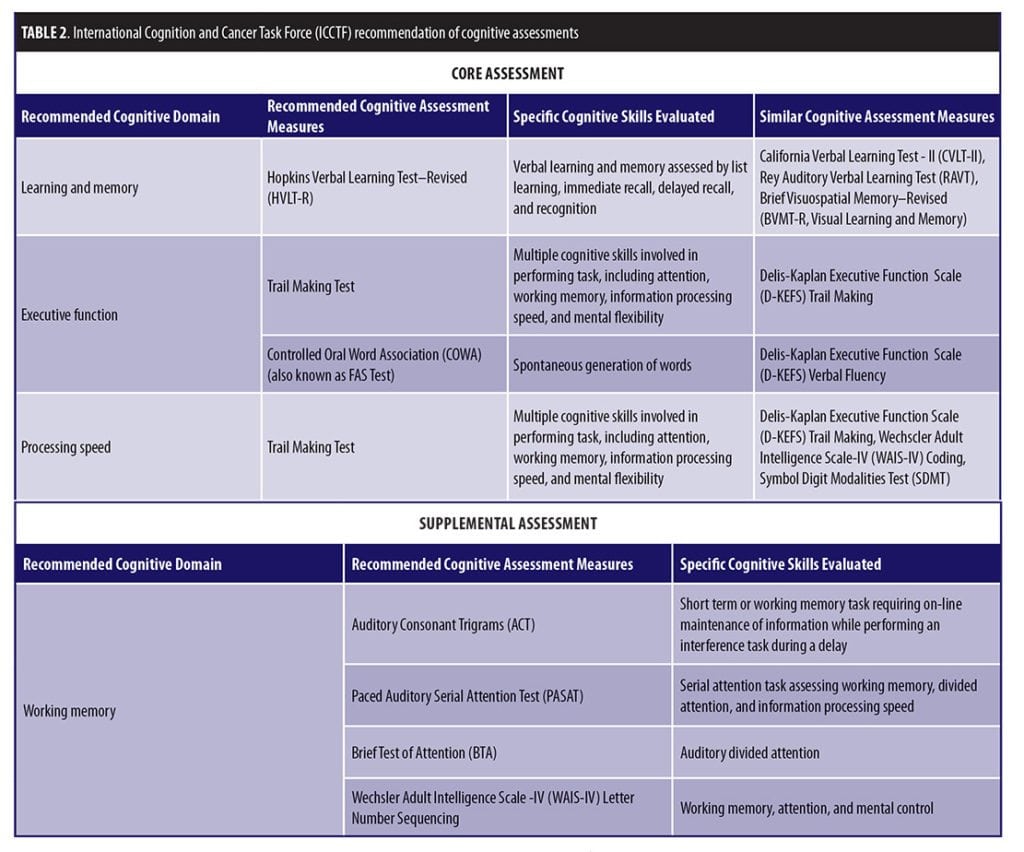
The International Cognition and Cancer Task Force (ICCTF) was developed in October 2006 with the goal of furthering the understanding of the impact that cancer and cancer-related treatment can have on cognitive and behavioral functioning in adults with non-CNS cancers.[89] The ICCTF has noted that, “objective neuropsychological tests remain the gold standard for measuring cognitive function” in the assessment of cognitive effects of cancer and cancer treatments.[3,6] The ICCTF organized two working groups that subsequently published recommendations for common criteria to define cognitive impairment and cognitive changes, as well as specific suggestions for a core set of cognitive tests to be used to assess cognitive function in patients with cancer (Table 2).[3] It is always prudent to approach standardization of cognitive tests cautiously because previous efforts for specific conditions (e.g., Alzheimer’s disease) have yielded sub-optimal solutions.90,91 However, the ICCTF group suggested that a collaborative approach that can leverage data across studies using common definitions and recommended a core set of cognitive tests for specific cognitive domains might yield a “best practice” guidance.[3,92–94] Careful attention must also be paid to issues of cross-cultural applicability and to assay sensitivity.[3,95]
Potential confounding factors that can negatively impact the assessment of cognitive functioning should also be evaluated in patients with cancer.[5] Common comorbid conditions like depression, pain, fatigue, and sleep disturbance might affect the reliability of the neuropsychological evaluation. In addition, all medications, including over-the-counter medications and supplements, should be reviewed to assess their possible impact on cognitive functioning.
COMPUTERIZED TESTING FOR ASSESSMENT OF COGNITIVE FUNCTIONING
Computerized testing has been adapted to assess cognitive function both in clinical trials with cancer patients and in practice.[24,79,96,97] Computerized cognitive assessments offer some logistical and economic advantages and flexibility over traditional pencil-and-paper cognitive tests. In this brief review, we describe three alternative computerized platforms that have been applied to cognitive assessment in cancer patients.
The CDR computerized system is a customizable battery of brief cognitive tests that has been used to assess the major cognitive domains identified as key for cancer-related treatments.[24,96–98] The standard CDR cognitive assessment battery includes immediate and delayed word recall, word recognition, picture recognition, simple reaction time, digit vigilance, choice reaction time, numeric working memory, and spatial working memory. Additional individual tests, such as executive function, can be added to the standard battery to target specific cognitive domains. The CDR demonstrated sensitivity in detecting cancer treatment-related cognitive changes in a clinical trial of patients with advanced colorectal cancer by revealing that patients treated with recombinant IL-2 (rIL-2) with chemotherapy experienced significant cognitive impairments, especially in reaction time, picture recognition, and vigilance compared to patients treated only with chemotherapy. Additionally, baseline cognitive functioning was restored within 10 days following the cessation of rIL-2.[96] In one study of adult patients with cancer (Hodgkin’s disease, non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, or multiple myeloma), the CDR found marked impairments on the Power of Attention domain (focused attention, information processing ability, and ability to concentrate), as well as slowing in speed of retrieval of information.[98] In studies of patients with breast cancer, the CDR system has recorded significant impairment in attention, verbal memory, visual recognition memory, and working memory.[24,78]
CNS Vital Signs is another a computerized neurocognitive test battery that has been validated in a broad age range and across both clinical and nonclinical populations, including cancer patients.[99] The CNS Vital Signs platform has 10 normed neurocognitive tests available, including verbal memory, visual memory, finger tapping, symbol digit coding, the Stroop test, shifting attention, continuous performance test, perception of emotions, nonverbal reasoning, and four-part continuous performance test. The 10 normed neurocognitive tests of CNS Vital Signs can be arranged into a custom testing panel for standardized data collection and can be complemented with additional 26 un-normed cognitive tests. In one prospective study examining the incidence and severity of cognitive dysfunction in meningioma patients before and after surgery, cognitive assessment using the CNS Vital Signs battery found that meningioma patients demonstrated significantly lower scores in all cognitive domains (i.e., memory, psychomotor speed, reaction time, complex attention, cognitive flexibility, processing speed, and executive functioning) pre- and post-operatively compared to normative data, which is consistent with previous research examining cognitive functioning in meningioma patients with conventional paper-and-pencil cognitive measures.[100] Additional analyses revealed that patients demonstrated improvement in test performance post-operatively in all domains, with the exception of psychomotor speed and reaction time. Evaluation of cognitive functioning using the CNS Vital Signs battery in lymphoma survivors who had completed treatment in the past five years revealed that lymphoma survivors obtained significantly poorer scores on measures of attention and executive functioning compared to controls.[32]
CogState is another computerized testing platform that offers a series of valid and reliable computerized neurocognitive tests that can be customized for cognitive assessment of cancer patients. The CogState Brief Battery assesses four core cognitive domains: processing speed, attention, visual learning, and working memory, and has been used extensively in evaluating cognitive functioning and impairments in a range of clinical indications in adult and pediatric populations. A recent study utilized the CogState Brief Battery Tests and conceptually matched traditional neuropsychological tests and a self-report measure of daily functioning (the Functional Activities Questionnaire) in 53 post-menopausal women (26 breast cancer survivors and 27 healthy controls).101 Analyses revealed significant correlations between the CogState Brief Battery tests on some, but not all, traditional neuropsychological tests.[101] These researchers reported preliminary support for criterion validity but noted additional research was needed to further confirm the use of CogState tests to detect subtle cognitive differences between breast cancer survivors and healthy controls, given the small sample size and low base rate of cognitive impairment in the study. In a separate, ongoing study collecting cerebrospinal fluid while prospectively assessing cognitive functioning in patients with acute lymphoblastic leukemia (ALL) during and after chemotherapy, baseline data found that 85 percent of patients demonstrated intact cognitive functioning in domains of working memory, executive functioning, learning, processing speed, and attention on the CogState computerized battery compared to same age peers along with the baseline cerebral spinal fluid (CSF) markers being within expected normal ranges.[102]
MANAGEMENT AND TREATMENT INTERVENTIONS FOR COGNITIVE CHANGES IN COGNITION
Some interventions might be useful to manage the cognitive changes experienced by patients with cancer. Possible nonpharmacological interventions include cognitive rehabilitation, occupational therapy, instruction in coping strategies, behavioral modification, and mindfulness practices to manage distress, pain, sleep disturbances, and fatigue.[5,103] Clinically it is usually appropriate to begin with nonpharmacologic interventions. For instance, attention retraining and instructional use of compensatory strategies have shown some promise in addressing cognitive complaints and mental fatigue in patients with cancer.[104] Similarly, cognitive rehabilitation and training techniques that employed repeated skills training, awareness practice, and adaptive difficulty levels showed positive benefits in cognitive organization and self-reported quality of life.[105] A multidisciplinary approach delivered by a team of psychologists, speech and language pathologists, and occupational and vocational specialists has been shown to facilitate improved community independence and employment outcomes in a study of patients diagnosed with brain tumors.[106]
Some of the neurotoxic fatigue and cognitive side effects of cancer chemotherapy might be managed with psychostimulant medications.[8] It is important to note that psychostimulants are generally prescribed for a limited period of time during chemotherapy and that the long-term benefits, if any, for cognition in survivors of cancer has not been assessed. Gehring et al[107] found that patients with brain tumors treated with methylphenidate or modafinil had improvements in divided attention, particularly in patients who had the greatest cognitive impairment at baseline. Using the CDR computerized test system, Kohli et al[97] found that modafinil 200mg given daily for eight weeks improved cognitive functioning in the domains of memory and attention in survivors of breast cancer, specifically the speed of memory and quality of episodic memory.
Donepezil also has been used to treat cancer-related fatigue, attention, and memory, although the data come from small studies and must be interpreted with caution.[108–111] A recent pilot trial of 15 patients with brain tumor(s) revealed significant post-baseline improvement in attention, graphomotor speed, visual memory, and self-reported quality of life after receiving a daily dose of donepezil for 24 weeks.[111] Lawrence et al[110] reported improved delayed verbal memory following donepezil treatment in patients with breast cancer, with self-reported cognitive impairments 1 to 5 years following adjuvant chemotherapy. Wefel et al[112] reported a delayed time to cognitive decline in patients with brain metastases undergoing WBRT who received memantine treatment compared to placebo.
CONCLUSION
Cognitive impairment can affect daily functioning, quality of life, and capacity to work in patients with cancer and those in remission. Consequently, cognitive assessment is now an important and necessary part of a comprehensive oncological care plan. Cancer-related cognitive impairment might be due to the direct effects of the cancer itself, nonspecific factors, or comorbid conditions that are independent of the disease and/or due to the adverse effects of the treatment or combination of treatments given for the disease. The prevalence and extent of cognitive impairment associated with cancer is recognized but not well understood due, in part, to marked differences in the research methods and definitions used for evaluating cognitive functioning in these patients. Most studies have identified attention, memory, and information processing as the most frequent cognitive domains impacted by cancer and cancer-related treatments, but further research clearly is needed. Recent efforts have been made to develop common definitions to define cognitive impairment in cancer patients and to suggest guidelines for the most appropriate cognitive tests to be used.
Cognitive function has increasingly been recognized as a requisite therapeutic target in many diseases, including cancer. The extent of cognitive impairment that is observed in patients with cancer and those in remission make cognitive functioning a particularly important target for clinical trials and clinical practice in oncology.
REFERENCES
- Weiss B. Chemobrain: a translational challenge for neurotoxicology. Neurotoxicology. 2008;29:891–898.
- Hodgson K, Hutchinson A, Wilson C, Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013;39:297–304.
- Wefel J, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–708.
- Janelsins M, Kesler S, Ahles T, Morrow G. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26:102–113.
- Denlinger C, Ligibel J, Are M, et al. Survivorship: cognitive function, version 1.2014: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2014;12:976–986.
- Moore H. An overview of chemotherapy-related cognitive dysfunction, or “chemobrain.” Oncology (Williston Park). 2014;28:797–804.
- Jean-Pierre P, Winters P, Ahles T, et al. Prevalence of self-reported memory problems in adult cancer survivors: a national cross-sectional study. J Oncol Pract. 2012;8:30–34.
- Bradshaw M, Wefel J. The neuropsychology of oncology. In: Parsons M, Hammeke T (eds). Clinical Neuropsychology: A pocket handbook for assessment, Third edition. Washington DC: American Psychological Association;2014:313–337.
- Wefel J, Patwardhan S, Strange C. Concurrent and criterion validity related evidence for the neurocognitive function clinical trial battery in brain tumor patients. Presented at the 15th Annual Meeting of the Society for Neuro-Oncology; 2010 November 18; Montreal, Quebec.
- Meyers C, Smith J, Bezjack A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncology. 2004;12:157–165.
- Gehring K, Aaronson N, Taphoorn M, Sitskoorn M. Interventions for cognitive deficits in patients with a brain tumor: an update. Expert Rev Anticancer Ther. 2010;10:1779–1795.
- Tucha O, Smely C, Preier M, Lange K. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47:324–333.
- Klein M, Taphoorn M, Heimans J, et al. Neurobehavioral status and healthrelated quality of life in newly diagnosed highgrade glioma patients. J Clin Oncol. 2001;19:4037–4047.
- van Nieuwenhuizen D, Klein M, Stalpers LJ, et al. Differential effect of surgery and radiotherapy on neurocognitive functioning and healthrelated quality of life in WHO grade I meningioma patients. J Neurooncol. 2007;84:271–278.
- Aaronson N, Taphoorn M, Heimans J, et al. Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol. 2011;29:4430–4435.
- van Loon E, Heijenbrok-Kal M, van Loon W, et al. Assessment methods and prevalence of cognitive dysfunction in patients with low-grade glioma: a systematic review. J Rehabil Med. 2015;47:481–488.
- Lageman S, Cerhan J, Locke D, et al. Comparing neuropsychological tasks to optimize brief cognitive batteries for brain tumor clinical trials. J Neurooncol. 2010;96:271–276.
- Meyers C, Albitar M, Etsey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia and myelodysplastic syndrome. Cancer. 2005;104:788–793.
- Ahles T, Saykin A, McDonald B, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143–152.
- Wefel J, Saleeba A, Buzdar A, Meyers C. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356.
- Jansen C, Cooper B, Dodd M, Miaskowski C. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19:1647–1656.
- Wefel J, Vidrine D, Veramonti T, et al. Cognitive impairment in men with testicular cancer prior to adjuvant therapy. Cancer. 2010;117:190–196.
- Tager F, McKinley P, Schnabel F, et al. The cognitive effects of chemotherapy in post-menopausal breast cancer patients: a controlled longitudinal study. Breast Cancer Res Treat. 2010;123:25–34.
- Walker L, Walker M, Marshall I, et al. Cognitive function in breast cancer patients compared with age matched healthy controls. J Psychopharmacology. 2002;16:A27.
- Hurria A, Rosen C, Hudis C, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. J Am Geriatric Soc. 2006;54:925–931.
- Wefel J, Lenzi R, Theriault R, et al. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–2299.
- Berman M, Askren M, Jung M, et al. Pretreatment worry and neurocognitive responses in women with breast cancer. Health Psychology. 2014;33:222–231.
- Hermelink K, Kuchenhoff H, Untch M, et al. Two different sides of ‘chemobrain’: determinants and nondeterminants of self-perceived cognitive dysfunction in a prospective, randomized, multicenter study. Psycho-Oncology. 2010;19:1321–1328.
- Koppelmans V, Breteler M, Boogerd W, et al. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30:1080–1086.
- Biglia N, Bounous V, Malabaila A, et al. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: a prospective study. Eur J Cancer Care. 2012;21:485–492.
- Castellon S, Ganz P, Bower J, et al. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26:955–969.
- Krolak D, Collins B, Weiss L, et al. Cognitive function and its relationoship to other psychosocial factors in lymphoma survivors. Support Care Cancer. 2017;25:905–913.
- Brown P, Ballman K, Rummans T, et al. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncol. 2006;76:283–291.
- Osoba D, Brada M, Prados M, et al. Effect of disease burden on health-related quality of life in patients with malignant gliomas. Neuro Oncol. 2000;2:221–228.
- Schagen S, Das E, Vermeulen I. Information about chemotherapy-associated cognitive problems contributes to cognitive problems in cancer patients. Psychooncology. 2012;21:1132–1135.
- Ahles T, Saykin A, McDonald B, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440.
- Collins B, Mackenzie J, Stewart A, et al. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009;18:134–143.
- Boykoff N, Moieni M, Subramanian S. Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv. 2009:3;223–232.
- Lindner O, Phillips B, McCabe M, et al. A meta-analysis of cognitive impairment following adult cancer chemotherapy. Neuropsychology. 2014;28:726–740.
- Jim H, Phillips K, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard dose chemotherapy. J Clin Oncol. 2012;30:3578–3587.
- Tchen N, Juffs H, Downie F, et al. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol. 2003;21:4175–4183.
- Ganz P, Kwan L, Castellon S, et al. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013;105:791–801.
- Ahles T, Saykin A. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201.
- Ahles T, Root J, Ryan E. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30:3675–3686.
- Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30:274–281.
- Deprez S, Billiet T, Sunaert S, Leemans A. Diffusion tensor MRI of chemotherapy-induced cognitive impairment in non-CNS cancer patients: a review. Brain Imaging Behav. 2013;7:409–435.
- Simo M, Rifa-Ros X, Rodriguez-Fornells A, Bruna J. Chemobrain: a systematic review of structural and functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37:1311–1321.
- Shah R. Mechanistic basis of adverse drug reactions: The perils of inappropriate dose schedules. Expert Opin Drug Saf. 2005;4:103–128.
- Sul J, DeAngelis L. Neurologic complications of cancer chemotherapy. Seminars Oncoloy. 2006;33:324–332.
- Ahles T. Brain vulnerabilities to chemotherapy toxicities. Psychooncology. 2012;21:1141–1148.
- Tonelli L, Postolache T, Sternberg EM. Inflammatory genes and neural activity: involvement of immune genes in synaptic function and behavior. Front Biosci. 2005;10:675–680.
- Tsavaris N, Kosmas C, Vadiaka M, et al. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87:21–27.
- Pusztai L, Mendoza T, Reuben J, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102.
- Penson R, Kronish K, Duan Z, et al. Cytokines IL-1?, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNF? in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Cancer. 2000;10:33–41.
- Trask P, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000;18:2316–2326.
- Scheibel R, Valentine A, O’Brien S, Meyers C. Cognitive dysfunction and depression during treatment with interferon- and chemotherapy. J Neuropsych Clin Neurosci. 2004;16:185–191.
- Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-? treatments in cancer patients. Psychosom Med. 2001; 63:376–386.
- Verstappen C, Heimans J, Hoekman K, Postma T. Neurotoxic complications of chemotherapy in patients with cancer: Clinical signs and optimal management. Drugs. 2003;63:1549–1563.
- Sedletska Y, Giraud-Panis M, Malinge J. Cisplatin is a DNA-damaging antitumor compound triggering multifactorial biochemical responses in cancer cells: Importance of apoptotic pathways. Curr Med Chem Anticancer Agents. 2005;5:251–265.
- Rolig R, McKinnon P. Linking DNA damage and neurodegeneration. Trends Neurosci. 2000;23:417–424.
- Blasiak J, Arabski M, Krupa R, et al. Basal, oxidative and alkylative DNA damage, DNA repair efficacy and mutagen sensitivity in breast cancer. Mutat Res. 2004;554:139–148.
- Nadin S, Vargas-Roig L, Drago G, et al. DNA damage and repair in peripheral blood lymphocytes from healthy individuals and cancer patients: a pilot study on the implications in response to chemotherapy. Cancer Lett. 2006;239:84–87.
- Wardell T, Ferguson E, Chinnery P, et al. Changes in human mitochondrial genome after treatment of malignant disease. Mutat Res. 2003;525:19–27.
- Weijl N, Elsendoorn T, Moison R, et al. Non-protein bound iron release during chemotherapy in cancer patients. Clin Sci. 2004;106:475–484.
- Kaya E, Keskin L, Aydogdu I, et al. Oxidant/antioxidant parameters and their relationship with chemotherapy in Hodgkin’s lymphoma. J Int Med Res. 2005;33:687–692.
- Papageorgiou M, Stiakaki E, Dimitriou H, et al. Cancer chemotherapy reduces plasma total antioxidant capacity in children with malignancies. Leukemia Res. 2005;29:11–16.
- Kennedy D, Ladas E, Rheingold S, et al. Antioxidant status decreases in children with acute lymphoblastic leukemia during the first six months of chemotherapy treatment. Ped Blood Cancer. 2005;44:378–385.
- Goode E, Ulrich CM, Potter J. Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1513–1530.
- Hung R, Hall J, Brennan P, Boffetta P. Genetic polymorphisms in the base excision repair pathway and cancer risk: A HuGE review. Am J Epidemiol. 2005;162:925–942.
- McDonald B, Conroy S, Ahles T, et al. Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast Cancer Res Treat. 2010;123: 819–828.
- Kesler S, Bennett F, Mahaffery M, Spiegel D. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clin Cancer Res. 2009;15:6665–6673.
- McDonald B, Conroy S, Ahles T, et al. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30:2500–2508.
- Bruno J, Hosseini S, Kessler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;48:329–338.
- Hosseini S, Koovakkattu D, Kesler S. Altered small-world properties of gray matter networks in breast cancer. BMC Neurol. 2012;12:28.
- Kesler S, Wefel J, Hosseini S et al. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc Natl Acad Scie USA. 2013;110:11600–11605.
- Phillips K, Jim H, Small B, et al. Cognitive functioning after cancer treatment: a 3-year longitudinal comparison of breast cancer survivors treated with chemotherapy or radiation and noncancer controls. Cancer. 2012;118:1925–1932.
- Wefel J, Lenzi R, Theriault R, et al. “Chemobrain” in breast cancer? a prologue. Cancer. 2004;101:466–475.
- Rodgers J, Morse R, Verrill M, et al. Cognitive functioning in women following adjuvant treatment for breast cancer. J Psychopharmacol. 2003;17:A68.
- Jenkins V, Shilling V, Deutsch G, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer. 2006; 94: 828–34.
- Sof?etti R, KocherM, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31:65–72.
- Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol. 2009;27(1):78–84.
- Chang E, Wefel J, Hess, K, et al. Neurocognition in patients with brain metastases treated radiosurgery or radiosurgery plus whole-brain irradiation: A randomized controlled trial. Lancet Oncology. 2009;10:1037–1044.
- Crossen J, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: A review of radiation induced encephalopathy. J Clin Oncol. 1994;12:627–642.
- Merchant T, Conklin H, Wu S, et al. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–3697.
- Gondi V, Hermann B, Mehta M, Tome W. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2013;85:348–354.
- Sheline G, Wara W, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–1228.
- Meyers C, Brown P. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–1309.
- Wefel J, Witgert M, Meyers C. Neuropsychological sequelae of noncentral nervous system cancer and cancer therapy. Neuropsychol Rev. 2008; 18:121–131.
- Vardy J, Wefel J, Ahles T, et al. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol. 2008;19:623–629.
- Harrison J. Measuring cognitive change in Alzheimer’s disease clinical drug trials. J Nutr Health Aging. 2007;11:327–329.
- Harrison J, Minassian S, Jenkins L, et al. (2007). The NTB: A neuropsychological test battery for use in Alzheimer’s disease clinical trials. Arch Neurol. 2007; 64:1323–1329.
- Harrison JE, Lophaven S, Olsen CK. Which cognitive domains are improved by treatment with vortioxetine? Intl J Neuropsychopharm. 2016;19:1–6.
- Ritchie K, Ropacki M, Albala B, et al. Recommended cognitive outcomes in pre-clinical Alzheimer’s disease: consensus statement from the European Prevention of Alzheimer’s Dementia (EPAD) Project. Alzheimer’s and Dementia. 2016;13:186–195.
- Harrison J. Measuring the mind: Detecting cognitive deficits and measuring cognitive change in patients with depression. In: McIntyre R, Cha D (eds.) Cognitive Impairment in Major Depressive Disorder. Cambridge: Cambridge University Press;2016.
- Harrison J, Lam R, Baune B, McIntyre R. Selection of cognitive tests for trials of therapeutic agents.Lancet Psychiatry. 2016;8:1–13.
- Walker L, Wesnes K, Heys S, et al. The cognitive effects of recombinant interleukin-2 (rIL-2) therapy: a controlled clinical trials using computerized assessments. E Jrnl Cancer. 1996;32A:2275–2283.
- Kohli S, Fisher S, Tra Y, et al. The effect of modafinil on cognitive function in breast cancer survivors. Cancer. 2009;115:2605–2616.
Wesnes K, Edgar C, Brooke, H. The disruptions to cognition, everyday function and quality of life in oncology patients: a therapeutic opportunity. Presented at the 6th annual International Society for CNS Clinical Trials (ISCTM). 2010 February; Washington DC. - Gualtieri C, Johnson L. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006;21:623–643.
- Meskal I, Gehring K, van der Linden SD, et al. Cognitive improvement in meningioma patients after surgery: clinical relevance of computerized testing. J Neurooncol. 2015;121:617–625.
- Patel S, Meir A, Fernandez N, et al. Convergent and criterion validity of the CogState computerized brief battery cognitive assessment in women with and without breast cancer. Clin Neuropsychol. 2017;12:1–12.
- Sands S, Harel B, Savone M, et al. Feasibility of baseline neurocognitive assessment using Cogstate during the first month of therapy for childhood leukemia. Support Care Cancer. 2017;25:449–457.
- Chan R, McCarthy A, Devenish J, et al. Systematic review of pharmacologic and nonpharmacologic interventions to manage cognitive alterations after chemotherapy for breast cancer. Euro J Cancer. 2015;51:437–450.
- Gehring K, Roukema J, Sitskoorn M. Review of recent studies on intervention for cognitive deficits in patients with cancer. Expert Adv Anticancer Ther. 2012;12:255–269.
- Ferguson R, McDonald B, Rocque M, et al. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psychooncology. 2012;21:176–186.
- Sherer M, Meyers C, Bergloff P. Efficacy of postacute brain injury rehabilitation for patients with primary malignant brain tumors. Cancer. 1997;80:250–257.
- Gehring K, Patwardhan S, Collins R, et al. A randomized trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. J Neuro Oncol. 2012;107(1):165–174.
- Shaw E, Rosdhal R, D’Agostino R Jr, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24:1415–1420.
- Rapp S, Case L, Peiffer A, et al. Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol. 2015;33:1653–1659.
- Lawrence J, Griffin L, Balcueva E, et al. A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J Cancer Surviv. 2016:10:176–184.
- Correa D, Kryza-Lacombe M, Baser R, et al. Cognitive effectis of donepezil therapy in patients with brain tumors: a pilot study. J Neurooncol. 2016;127:313–319.
- Brown P, Pugh S, Laak N, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–1437.





