
by Antonino Naro, MD, PhD; Luana Billeri, MSc; Paola Lauria, MSc; Alfredo Manuli, MSc; and Rocco Salvatore Calabrò, MD
Drs. Naro and Calabrò, Ms. Billeri, Ms. Lauria, and Mr. Manuli are with IRCCS Centro Neurolesi Bonino Pulejo–Piemonte in Messina, Italy.
Funding: No funding was provided for this study.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Innov Clin Neurosci. 2022;19(1–3):15–18.
ABSTRACT: Patients with progressive multifocal leukoencephalopathy (PML) in the context of human immunodeficiency virus-acquired immunodeficiency syndrome (HIV-AIDS) show a partial improvement following rehabilitation; however, this improvement is rapidly lost if the patient is not provided with intensive rehabilitation. A 42-year-old patient affected by HIV-AIDS had a clinical worsening within a few months following PML onset, despite being treated with antiretroviral drugs and conventional rehabilitation. He developed severe paraparesis and significant dependency in the activities of daily life. A first cycle of intensive rehabilitation provided the patient with some significant functional outcomes, although he experienced a worsening of the clinical condition after two months of rest, before admission to our rehabilitation unit. We thus sought to evaluate the effects of intensive robot-aided gait training (RAGT) coupled with transcranial direct current stimulation (tDCS). The patient significantly improved when provided with intensive RAGT coupled with tDCS (as per 10-meter Walk Test [10MWT] and 6-minute Walk Test [6MWT]), and the improvement was maintained at three-month follow-up. As this advanced approach was feasible, safe, and potentially effective, this case suggests that patients with PML-HIV require prolonged multidisciplinary rehabilitation treatment. We can speculate that individuals with PML should also be treated with innovative technology to improve their functional outcomes and therefore quality of life.
Keywords: Progressive multifocal leukoencephalopathy, human immunodeficiency virus, robot-aided gait training, transcranial direct current stimulation
Progressive multifocal leukoencephalopathy (PML) is a rare, severe, immune-mediated, demyelinating disorder of the central nervous system (CNS) caused by human polyomavirus JC (JCV), having tropism for the oligodendrocytes. After asymptomatic infection in childhood, JCV remains quiescent in the kidneys, bone marrow, and lymphoid tissues.1 In the setting of immunosuppression, the virus can reactivate, whereupon it can migrate into the brain, where genetic changes occur (neuroadaptation), allowing replication in the glial cells with PML development. PML has emerged as a major complication of immunosuppressive treatments (for organ transplants, cancer, or autoimmune diseases) and human immunodeficiency virus (HIV) infection. Furthermore, the highly active antiretroviral therapy (HAART), while dramatically reducing the PML risk, can sometimes further worsen PML, owing to an autoimmune response fostered by the restoration of CD4+ T cells count that leads to the PML-immune reconstitution inflammatory syndrome (IRIS).2
The acquired immunodeficiency syndrome (AIDS)-associated classical PML is typically seen when CD4 cell count drops below 200 cells/μL (also above 200 in case of PML-IRIS), and it is characterized by demyelination, astrocyte modifications, and oligodendroglial nuclei changes, while edema, lymphocyte infiltration, and blood-brain barrier damage are typically absent.3 This pattern causes altered mental status motor deficits, limb and gait ataxia, and visual symptoms (diplopia and hemianopia). Neuroimaging data (confluent, bilateral but asymmetrical demyelination areas within occipital, frontal, and parietal lobes, cerebellar peduncles, and deep white matter; spinal cord involvement is rare) and cerebrospinal fluid (CSF) molecular biology testing (CSF JCV detection by polymerase chain reaction [PCR]) confirm the diagnosis of PML.
The management of patients with AIDS-related PML is challenging, as there is no specific anti-JCV treatment. Although HAART has significantly improved the prognosis of patients with AIDS-related PML, the percentage of patients with sequelae is not negligible, and these patients require an intensive rehabilitative program to recover cognitive and sensorimotor impairment and to limit disability burden.4,5
In this regard, it is worthy to note that PML outcomes depend entirely on the individual’s capacity to recover immune system function and respond to JCV and, in parallel, to the neuronal plasticity processes that are fundamental to recover lost brain function.6 Therefore, this issue cannot be neglected in the case of PML. In this context, noninvasive brain stimulation (NIBS) techniques, including transcranial direct current stimulation (tDCS), have been proposed as promising to entrain the complex neuroplasticity-neuroimmune mechanisms of recovery.7,8
Thus, we applied an NIBS protocol using double tDCS during intensive robot-aided gait training (RAGT) to a patient with AIDS-related PML presenting with severe paraparesis.
Case Description
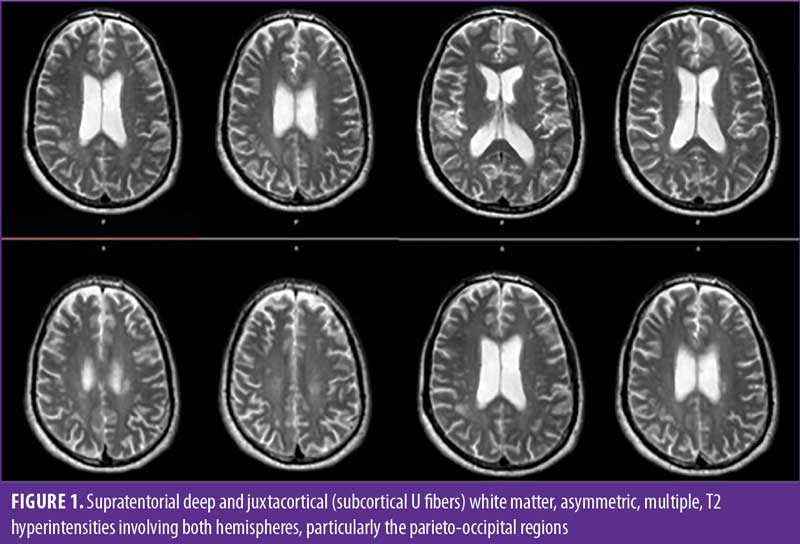
A 42-year-old man was admitted to our Neurorobotics Rehabilitation Unit in 2019 to undergo intensive rehabilitation owing to a severe paraparesis. He was diagnosed with HIV about seven years prior to admission and was treated with emtricitabina and tenofovir. About six months before our observation, he was admitted to the emergency room (ER) due to confusion, dysarthria, and decreased sensation and strength on the lower limbs with gait ataxia, which progressively led to a paraparesis. He was hospitalized in a neurological unit where he underwent brain and spinal magentic resonance imaging (MRI), which documented multifocal, asymmetric periventricular and subcortical high T2 signal lesions, without enhancement (Figure 1). The CD4 cell count was 168 cells/mm3, with a viral load of 80,000 copies/mL. The CSF analysis showed 2 lymphocytes/mL, and 340mg/L protein, as well as a positive JCV-PCR, confirming the diagnosis of PML. He was thus prescribed darunavir, eviltegravir, cobicistat, emtricitabina, and tenofovir and was transferred to a rehabilitation unit to receive gait rehabilitation for the persistent severe paraparesis.
Upon admission, the patient’s lower limb strength was 2/5 at the thigh, 1/5 at the knee, and 1/5 distally. His Functional Independence Measure (FIM) was 78. The Berg Balance Scale (BBS), the 10-meter Walk Test (10MWT), and the 6-minute Walk Test (6MWT) were not administrable. Somatosensory and motor evoked potentials from lower limbs were clearly reduced in amplitude, with an increase in central motor conduction time. He received an inpatient intensive rehabilitation treatment with conventional physiotherapy and occupational therapy for two months. At discharge, lower limb strength was 3/5 at the thigh, 2/5 at the knee, and 2/5 distally. His FIM scoring was 95. The BBS (first 7 items, as he was not able to perform the test without support) was 12/28, the 10MWT was 25 seconds (with two elbow crutches, two ankle-foot orthoses [AFO], and supervision), and the 6MWT was not administrable. The American Spinal Injury Association Impairment Scale (AIS) grade was D.
After two months of rest, his condition significantly worsened. He was thus admitted to our rehabilitation unit to start an intensive rehabilitation regimen. Upon admission, lower limb strength was 3/5 at the thigh, 2/5 at the knee, and 1/5 distally. His FIM scoring was 84. The BBS (first 7 items) was 5/28, the 10MWT was 37 seconds, and the 6MWT was not administrable. Moreover, he had impairment in memory, attention, and delayed recall items of Montreal Cognitive Assessment (MOCA; 22/30).
He was provided two-hour daily sessions of conventional rehabilitation, including physical therapy, targeting muscle tone and strength, gait pattern, and symmetry, and occupational therapy, targeting independence in basic activities of daily living, the reaching of targets, and cognitive abilities, with an emphasis on visuomotor function, attention, and memory. Furthermore, he underwent one-hour daily sessions of RAGT (LokomatPro® Hocoma AG; Volketswil, Switzerland) six days per week for two months. He received daily sessions of tDCS, which preceded RAGT. Written informed consent was obtained from the patient for publication of this case report.
RAGT training. The RAGT device consists of a powered gait orthosis with integrated computer-controlled linear actuators at each hip and knee joint, a body weight support (BWS), and a treadmill. Moreover, it provided augmented performance feedback, which gives motivating, challenging, and instructive functional feedback in virtual environments by observing a human avatar walking on the screen. The rehabilitation training is designed to be more motivating, as the patient is aware of the performance and results achieved.
At the beginning of each RAGT session, the patient underwent a 10-minute period of fitting to the RAGT device to ensure that he was comfortable with the exoskeleton (correct fit of straps and cuffs, alignment set within a tolerable range of movement, adaption to RAGT device parameters, time required to reach the daily maximally tolerated BWS provided, walking duration, ambulation velocity, and device guidance force [DGF] provided to each leg), according to the user manual. Then, the gait training session began. The parameters of the RAGT device were progressively adapted to the patient’s tolerance, reaching the maximally tolerated walking duration (from 15–45 minutes), BWS (from 80–30%), ambulation velocity (from 0.9–1.3 m/s), and DGF (from 90–45%) during the first eight sessions. If the patient was unable to keep up with any of the progressions during any session, adjustments were carried out to a lesser extent or reversed. In each session, the patient was required to walk straight, pass obstacles, or catch objects appearing on the trail, thus being forced to change walking direction. These exercises were provided in a random order during each session. This motor strategy allowed for encouragement of lower limb selective muscle/joint activation and motor learning. The use of the RAGT device’s virtual reality games and the provision of visual biofeedback in each session also permitted encouraged engagement and promoted feedback. A physiotherapist trained in the use of the RAGT device supervised each session and monitored vital signs and exertion.
Neuromodulation. Neuromodulation consisted of a double tDCS, with the anode located over the motor cortex representation of the more-affected leg (C4 on the International Electroencephalogram [EEG] System), and the cathode was placed on the contralateral motor cortex; the reference electrodes were put on the respective contralateral supraorbital area. These electrodes were put into a sponge surface electrode (25cm2, soaked with 15mM NaCl) and wired to two transcranial stimulator devices (BrainStim, EMS; Bologna, Italy). The stimulation involved a 30-second ramp-up to 2mA, after which the intensity stayed at 2mA for the duration of the stimulation period (15 minutes). At the end of the stimulation time, the intensity was ramped down to 0mA over 30 seconds. Each neuromodulation session preceded RAGT practice.
Post-combined protocol outcomes. At discharge three months later, lower limb strength was 4/5 at the thigh, 3/5 at the knee, and 2/5 distally. He was able to walk with two AFO and one elbow crutch for intermediate distances. He was also able to climb up- and downstairs with a rail and the help of a caregiver. The BBS was 19/28, the FIM was 103, the 10MWT was 20 seconds, and the 6MWT was 144m (with two AFO, one elbow crutch, and no supervision). Moreover, he showed an improvement in MOCA scoring (27/30).
Owing to the SARS-CoV-2 pandemic, we were only able to conduct a telephone follow-up three months after discharge. Based on this information, his functional status was estimated as essentially unchanged.
Discussion
To the best of our knowledge, this is the first report on the use of tDCS and RAGT in the context of rehabilitation of patients with HIV-induced PML, though some aspects of rehabilitation of patients with HIV have been formerly addressed.4,5
Our patient had progressive clinical worsening within a few months from the clinical onset of PML, with severe paraparesis and significant dependency in the activities of daily life, despite the HAART and the rehab management. The first cycle of intensive, conventional rehabilitation provided the patient with some significant functional outcomes, but a clinical worsening occurred after two months of rest, prior to the admission to our rehabilitation unit. These data are consistent with the functional outcome of HIV-positive PML survivors, showing a partial improvement following rehabilitation that is rapidly lost if the patient is not provided with lasting, intensive rehabilitation.8 Conversely, the patient more evidently improved in gait velocity and endurance (which was never achieved earlier) and balance when he received the intensive rehabilitation training coupled with tDCS, compared to the former training period. Furthermore, the improvement was maintained at the three-month follow-up.9
The advanced approach was feasible, safe, and effective, suggesting that patients with PML should be treated with innovative technology to improve their functional outcomes and therefore quality of life.
The exact mechanism underpinning this important functional recovery is not clear, although neuromodulation might have played a pivotal role. It is known that tDCS can lastingly modify motor cortex excitability in a polarity-specific manner (i.e., depending on the current flow) by inducing long-term potentiation and depression-like plasticity mechanisms, particularly via NMDA-receptors.10–11
Furthermore, tDCS facilitates an increase in brain-derived neurotrophic factor (BDNF)-secretion, which might be another pathway sustaining tDCS after-effect.12
In parallel, tDCS has been experimentally proven to evoke various neurobiological effects on microglia, beyond its primary action on neurons. Particularly, tDCS can modulate immune function through a downregulation of inflammatory cytokine release, increase the proliferation and migration of endogenous neural stem cells and activate microglia as the brain-resident immune cells, and induce neurogenesis.13,14 Altogether, these immunomodulatory effects might support the well-known tDCS-induced recovery processes and stem cell-mediated regeneration in the brain, which develop from days to weeks after lasting stimulation paradigms.
The functional outcomes the patient achieved could be due to a combination of the neurorobotics and tDCS after-effects. The corticomotor excitability increase after tDCS could be synergistically potentiated by the robotic practice-induced neural plasticity, consistently with a similar phenomenon involving tDCS and motor training and according to the associative plasticity principles.15
Lastly, the patient also had cognitive improvements following the RAGT plus tDCS approach. Even though this issue deserves further confirmation, these cognitive effects suggest a wide-range effectiveness of tDCS and RAGT on brain function recovery.16–17
Limitations. Our case has two main limitations. First, the improvement we achieved might depend on the RAGT alone, and not necessarily on the addition of tDCS, compared to the previous conventional rehabilitation. Second, the patient was not provided with a sham-tDCS. However, the degree and, particularly, the duration of improvement was consistently greater following the coupled approach than the conventional approach, which suggests a real contribution of tDCS on clinical outcomes. Notwithstanding, further studies are necessary to confirm the real usefulness of tDCS as add-on therapy to RAGT. One might argue that this consistent improvement might depend on a summation effect between conventional treatment and RAGT plus tDCS approaches, but the two-month interval period between the two trainings makes this concern unlikely.
Conclusion
Regardless of the mechanism of tDCS-RAGT interaction, our case suggests that a multidisciplinary, high-intensity rehab protocol might be the best viaticum for functional recovery in the case of HIV-PML. Furthermore, we argue that providing patients affected by HIV-PML with neuromodulation could be useful to improve and prolong functional outcome achievement. However, further cases and more objective measures are required to confirm our promising findings and to better clarify the neurophysiological underpinnings of the clinical improvement.
References
- Brew B, Davies N, Cinque P, et al. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol. 2010;6(12):667–679.
- Pavlovic D, Patera AC, Nyberg F, et al. Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord. 2015;8(6):255–273.
- Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80(15):1430–1438.
- Moreh E, Israel S, Korem M, Meiner Z. Rehabilitation outcome of progressive multifocal leukoencephalopathy in HIV-positive patients: a report of two cases. Disabil Rehabil. 2017;39(18):1893–1896.
- O’Brien KK, Solomon P, Trentham B, et al. Evidence informed recommendations for rehabilitation with older adults living with HIV: a knowledge synthesis. BMJ Open. 2014;4:e004692.
- De Luca C, Colangelo AM, Alberghina L, Papa M. Neuro-immune hemostasis: homeostasis and diseases in the central nervous system. Front Cell Neurosci. 2018;12:459.
- Cullen CL, Young KM. How does transcranial magnetic stimulation influence glial cells in the central nervous system? Front Neural Circuits. 2016;10:26.
- Lima MA, Bernal-Cano F, Clifford DB, et al. Clinical outcome of long-term survivors of progressive multifocal leukoencephalopathy. J Neurol Neurosurg Psychiatry. 2010;81(11):1288–1291.
- Uygur-Kucukseymen E, Fregni F. Repetitive transcranial magnetic stimulation (TMS): does the stimulation of the brain cortex modulate the immune system? In: Fregni F, ed. Looking for the Magic Pill: The Anti-inflammatory Brain: The new science of nervous system modulation to reduce inflammation. 2020:27–42.
- Monte-Silva K, Kuo MF, Hessenthaler S, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated noninvasive brain stimulation. Brain Stimul. 2013;6(3):424–432.
- Kronberg G, Bridi M, Abel T, et al. Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain Stimul. 2017;10(1):51–58.
- Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56–92.
- Braun R, Klein R, Walter HL, et al. Transcranial direct current stimulation accelerates recovery of function, induces neurogenesis and recruits oligodendrocyte precursors in a rat model of stroke. Exp Neurol. 2016;279:127–136.
- Pikhovych A, Stolberg NP, Jessica Flitsch L, et al. Transcranial direct current stimulation modulates neurogenesis and microglia activation in the mouse brain. Stem Cells Int. 2016; 2016:1–9.
- Edwards DJ, Krebs HI, Rykman A, et al. Raised corticomotor excitability of M1 forearm area following anodal tDCS is sustained during robotic wrist therapy in chronic stroke. Restor Neurol Neurosci. 2009;27(3):199–207.
- Maggio MG, Naro A, La Rosa G, et al. Virtual reality based cognitive rehabilitation in minimally conscious state: a case report with EEG findings and systematic literature review. Brain Sci. 2020;10(7):414.
- Yamaguchi T, Moriya K, Tanabe S, et al. Transcranial direct-current stimulation combined with attention increases cortical excitability and improves motor learning in healthy volunteers. J Neuroeng Rehab. 2020;17(1):23.





