 by Vishwanath Sagi MBBS, MPH; Jaime Shoup MD; Ravikiran Chilukuri; and M. Steven Evans MD
by Vishwanath Sagi MBBS, MPH; Jaime Shoup MD; Ravikiran Chilukuri; and M. Steven Evans MD
Drs. Sagi and Evans are with the Department of Neurology at the University of Louisville in Louisville, Kentucky. Drs. Shoup and Chilukuri are with the University of Louisville School of Medicine in Louisville, Kentucky.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. The objective was to study latency to first event among patients with psychogenic nonepileptic seizures compared (PNES) to epileptic seizures (ES) in an epilepsy monitoring unit (EMU).
Introduction. PNES are common imitators of ES. This study investigates latency to first event in patients with PNES compared to patients with ES.
Methods. We performed a retrospective chart review of patients admitted to our EMU from March 2016 to October 2017. We identified patients with PNES and ES. Patients with other nonepileptic events and mixed PNES (epilepsy plus PNES) were excluded. Patient demographics, baseline seizure frequency, length of EMU stay and time from admission to first event were recorded.
Results. In total, 111 patients with PNES and 121 patients with ES were included. The mean age (in years) was 42 and 38, respectively. The average baseline seizure frequency was four times higher in the PNES group than the ES group. Greater than half (52%) of the patients with PNES and about one third (38%) of the patients with ES had an event within the first 24 hours. The average time to first event was 20.88 hours for the PNES group and 30.99 hours for the ES group (p<0.01). The median latency to first event was 14 hours for the PNES group and 23 hours for the ES group. The average length of EMU stay was significantly longer in the ES group (70.82 hours) than the PNES group (53.95 hours).
Conclusion. The average time to first event is shorter for PNES than in ES. In patients with high pre-EMU clinical suspicion for PNES, relatively shorter EMU monitoring (24 to 48 hours) can confirm diagnosis. This phenomenon might improve cost-effectiveness of EMU monitoring in patients with PNES.
Keywords: Psychogenic nonepileptic events (PNES), epilepsy monitoring unit (EMU), latency to first event, epileptic seizures (ES), risk factors
Innov Clin Neurosci. 2020;17(7–9):26–29
Psychogenic nonepileptic seizures (PNES) are paroxysmal events or behaviors that resemble epileptic seizures (ES) but do not result from excessive neuronal activity.1,2 PNES account for 24 to 33 percent of patients evaluated in the epilepsy monitoring unit (EMU) for refractory seizures.3,4 Misdiagnosis and overmedication with antiepileptic drugs (AEDs) are common, and video electroencephalogram (VEEG) is essential for diagnosis.5,6 Multiple studies have already attempted to ascertain the optimal duration in the EMU in both ES and PNES.7–10 Friedman and Hirsch7 reported that most patients require at least three days of VEEG monitoring for spell capture and characterization. One study reported that most patients in the EMU have their first event within 48 hours but did not distinguish between PNES and ES.8 Another study compared latency to first event in EMU among different PNES subtypes.9 Foong et al10 compared ES and PNES time to first event and found no significant difference in median time, but also excluded patients with EMU duration 24 hours or less from their analysis. Our study was designed to determine latency to first event in patients with PNES compared to patients with ES in a large EMU database regardless of EMU duration. In addition, we analyzed historical characteristics, clinical profile, and use of psychiatric and antiepileptic medications in patients with PNES and ES.
Methods
This study was a retrospective chart review. After approval by the University of Louisville Institutional Review Board, we reviewed electronic patient medical records for EMU admission and progress notes, discharge summaries, and electroencephalogram (EEG) reports of patients admitted to the University of Louisville EMU from March 2016 to October 2017. EMU studies with less than 12 hours of recording time were excluded. In total, 654 patients were included. Only patients with a definitive diagnosis of either PNES or ES (based on discharge summary and EMU report) were included. Patients with mixed PNES and ES and patients with other nonepileptic causes of their events (cardiogenic syncope/sleep disorders) were excluded from analysis. In the PNES group, patients with interictal epileptiform abnormalities were excluded as well. In total, 111 patients were formally diagnosed with PNES at discharge, and 123 were found to have ES. Data on demographics, historical characteristics, and imaging were obtained from chart review. The diagnosis of anxiety, depression, or posttraumatic stress disorder (PTSD) was determined based on subjective patient report and/or chart review. We analyzed and compared length of stay, number of events in the EMU, time to first event after admission, pre-EMU seizure frequency, average number of AEDs, interictal EEG abnormalities, and average number of psychiatric medications between patients with PNES and patients with ES. Characteristics reported as frequencies were determined to have statistical significance using chi square analysis. Ratio data was first determined to be either normal distribution or nonparametric by plotting on histogram. Approximately normal distribution data, such as age at presentation or age of onset, was further analyzed using a 2-tailed t-test for comparison of means. Nonparametric data was analyzed using the Mann-Whitney U test. Chi squared contingency table analysis was additionally used to determine statistical significance in the distribution of time to first event and substance abuse rates.
Results
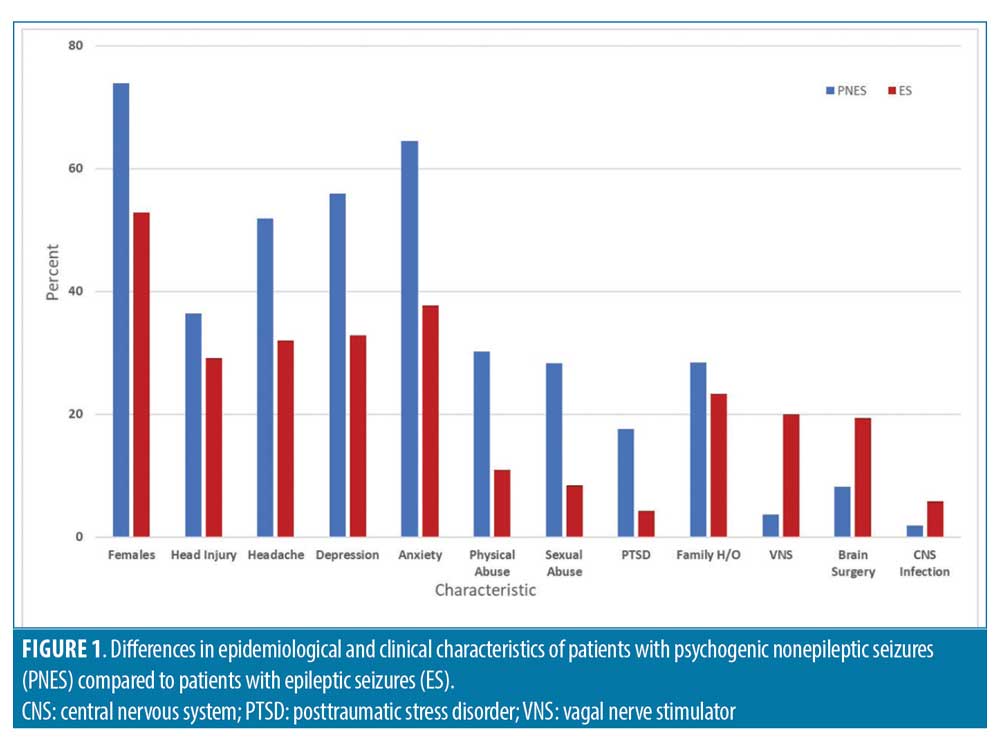
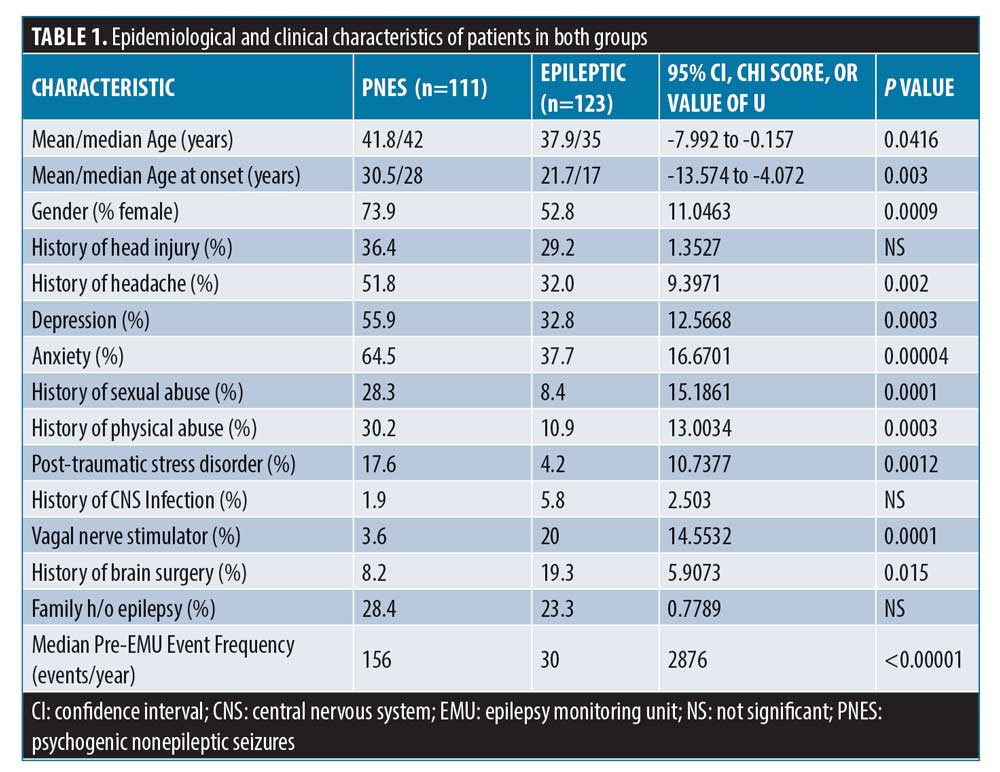
In total, 234 patients were reviewed with a mean age (years) of 40±15.1; 37.22 percent of patients were male and 62.8 percent were female. There were 82 (73.9%) female patients in the PNES group and 65 (52.8%) in the ES group (p=0.0009). The mean age (years) of seizure onset was 30.5 and 21.7, respectively. The median patient estimated pre-admission seizure frequency (events per year) was found to be five times greater among patients with PNES (156 vs. 30, p<0.00001). When risk factors were analyzed, 28.3 percent of patients with PNES reported history of sexual abuse compared to 8.4 percent of patients in the ES group (p=0.0001). History of physical abuse was also found to be significantly higher in patients with PNES (30.2% vs. 10.9%, p=0.0003). The difference in proportion of patients reporting head injury (36.4% vs. 29%) was not significant. Psychiatric comorbidities, such as depression, anxiety, and PTSD, were more prevalent in patients with PNES compared to patients with ES. Family history of epilepsy was higher in patients with PNES compared to ES (28.4% vs. 23.3%) but did not reach statistical significance. History of central nervous system (CNS) infection, vagal nerve stimulator (VNS), and brain surgery were higher in patients with ES compared to patients with PNES. Figure 1 and Table 1 summarizes the demographic and clinical characteristics of patients in both the groups.
The most common AEDs used in the PNES group were levetiracetam (34.2%), gabapentin (26.1%), and topiramate (20.7%), while the most common antiepileptic drugs for the epileptic group were levetiracetam (54.9%), lamotrigine (27.9%), and lacosamide (23.0%). The average number of antiepileptic drugs were 1.7 for the PNES group and 2.1 for the epileptic group. The most common psychiatric medications among patients with PNES were selective serotonin reuptake inhibitors (SSRIs) (31.5%), selective norepinephrine reuptake inhibitors (SNRIs) (11.7%), and atypical antipsychotics (9.9%). The most common psychiatric medications for the epileptic group were SSRIs (24.8%), atypical antipsychotics (7.4%), and SNRIs (6.6%). The average number of psychiatric drugs in patients with PNES and ES were 0.90 and 0.61, respectively.
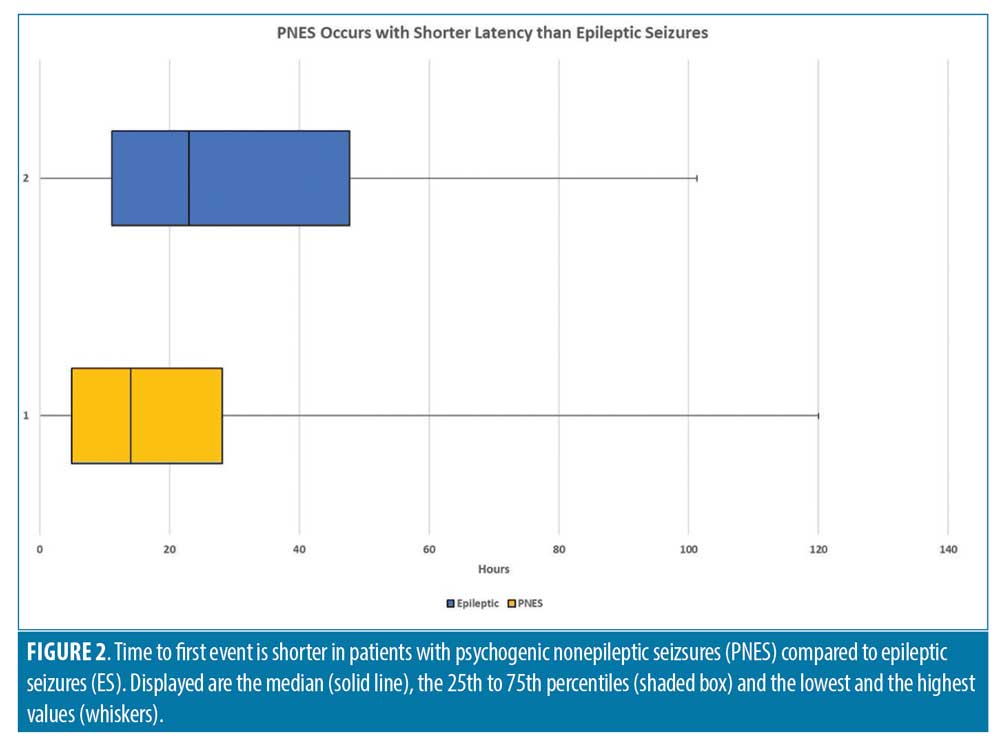
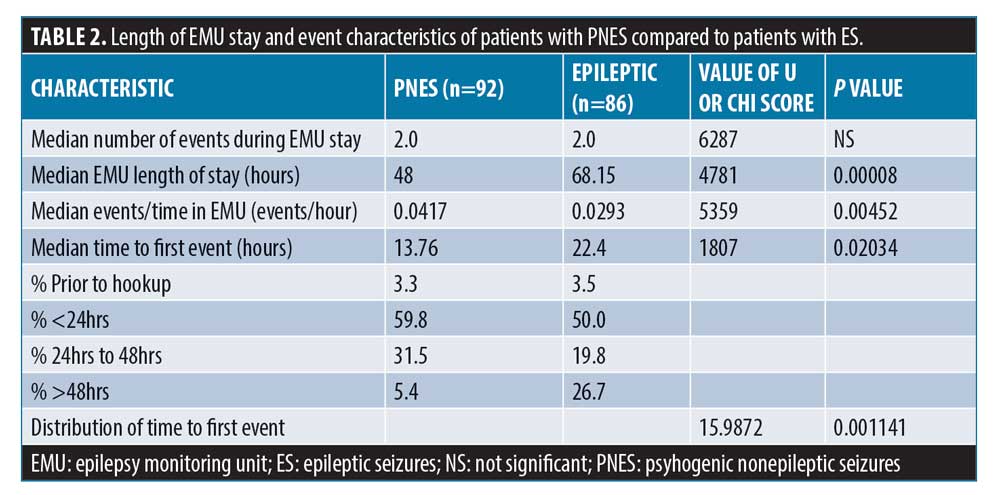
Data on precise timing of first event was available for 92 patients in the PNES group and 86 patients in the ES group. The mean time to first event was 20.8 hours in the PNES group compared to 31.0 hours in ES group. The median for time to first event was 13.76 hours for the PNES group, 22.4 hours for the ES group (Figure 2), and 18.6 hours for both groups combined. The percentage of patients with events in the first 24 hours was statistically higher in the PNES group (59.8% vs. 50.0%), and this difference increased further at 48 hours of monitoring (94.6% vs. 73.3%). The percentage of patients with greater than 48-hour latency to first event was higher in the ES group (26.7%) compared to the PNES group (5.4%). The overall differences in latency to first event were statistically significant using chi squared contingency table analysis (p=0.00011). On the other hand, patients with PNES had a shorter length of EMU stay (median 48 vs. 68.1, p=0.0008). Patients with PNES had a lower mean event rate (2.68 vs 4.16) and the median number of events was equal at 2. When the number of events per hour of EMU stay were analyzed, it did not reach statistical significance (p=0.2984). These differences are outlined in Table 2.
Discussion
This retrospective chart review compared risk factors and event characteristics in patients with PNES and ES in a large EMU database. We found a statistically significant difference in the median latency to first event between patients with PNES and patients with ES. In total, 95 percent of patients in the PNES group compared to 73 percent of patients in the ES group had their first event within 48 hours of admission. In addition, the PNES group had a five-times higher patient reported pre-admission event frequency compared to the ES group. These differences were statistically significant. No correlation was found between time to first event and reported pre-admission spell frequency. While the pre-EMU event frequency was significantly higher in patients with PNES, the total number of observed EMU events was higher in the group with epilepsy. This was likely due to longer lengths of stay in patients with epilepsy on average. When events were standardized for time, no difference in event frequency was observed in the EMU.
In this study, we confirmed the previous reports that most patients in the EMU have their first event within 48 hours of admission.11,12 Parra et al11 reviewed 100 consecutive patients admitted for VEEG monitoring. In their study, 78 percent and 96 percent of patients with PNES had their first event within 24 hours and 48 hours of monitoring, respectively. There were no significant differences in the number of events, gender, or age between the groups in that study as well. In contrast, our study found a significant female predominance (74%) in the PNES group compared to the ES group (53%). Finally, patients in the PNES group reported seizure onset at a later age than patients in the ES group (30.5 years vs. 21.7 years, p=0.003).
The baseline characteristics of our patient population are comparable to prior studies.10,13–16 The female predominance and significantly higher incidence of abuse among our patients with PNES have been shown previously.13 The prevalence of somatic symptoms in patients with PNES has also been previously reported. For instance, one study reported that 77 percent of patients with PNES experienced pain, most commonly headaches (61%).14 A similar pattern was noted in our population (52% of patients with PNES reported headache). The higher prevalence of headache might explain increased use of gabapentin and topiramate in our patients with PNES.
Multiple studies report increased rates of concomitant psychiatric diagnosis and PTSD with PNES, a trend also observed in our study.15–17 In our study, family history of epilepsy was reported more often in patients with PNES compared to patients with ES, which indicates that this historical detail does not distinguish between PNES and ES. Bautista et al18 examined whether the prevalence of having personally witnessed a seizure prior to the occurrence of their event differs in patients with PNES and those with epileptic seizures (symptoms modeling). The authors interviewed 27 patients with PNES and 35 patients with ES. In total, 66 percent of patients with PNES admitted to having witnessed a seizure prior to their event compared to 11 percent of patients with epileptic seizures (P<0.001). This symptom modeling might explain the suggestibility in our patients with PNES. In our study, head injury was also reported more often in patients with PNES compared to patients with ES. While there was a higher percentage of patients in the PNES group that suffered from both head injury and physical abuse compared to the patients with ES (16% vs. 5%), it is unclear if head injury is the result of the abuse, as the data relies solely on patient history and is not verified by another source.
Labello et al12 assessed the number of monitoring days needed to distinguish PNES from ES in patients with EMU. In their study, 88 percent (143/199) of patients had their first event within 48 hours of admission. The median duration to first event was 24 hours, but there was no association between time to first event and discharge diagnosis. Foong et al10 reported no significant difference in median time to first event between PNES and ES at the same EMU center but did not include patients with EMU duration less than 24 hours. More patients in the PNES group were found to also have EMU duration 24 hours or less in our study, which might explain discordance with their results. Another study found that nearly all events (98.5%) were captured within the first 48 hours, but also did not find a statistical difference of time to initial event.19 Cox et al8 also compared ES and PNES median time to first event and did not find a statistical significance either. The study, however, might have lacked sufficient power, as there were only 13 patients with PNES investigated, whereas 111 patients were included in this study. In comparison to these studies outlined, an important finding of our study was the statistically significant shorter median latency to first event in patients with PNES compared to patients with ES (13.76 hours vs. 22.4 hours, p=0.02034).
Several models exist on the phenomenology of PNES. The integrative cognitive model (ICM) described by Reuber et al,20 based on the theory of medically unexplained symptoms, consists of the observed and subjective aspects of PNES that result from automatic execution of a learnt mental presentation of seizures (seizures scaffold). The authors described the seizure scaffold as a broad “idea” of seizures consisting of a sequence of perceptions or motor activities formed by experiences such as startle reflexes, physical symptoms (e.g., head injury, syncope) and personal knowledge. It is possible that similar mechanisms contribute to the shorterlatency for patients with PNES in our study.20
The general practice in our EMU is to gradually decrease AED doses for patients with suspected seizures in the first 24 to 48 hours of EMU stay. Such interventions might precipitate PNES as a reaction to expectation (nocebo effect). In addition, prior studies emphasized suggestive effect of induction techniques (photic stimulation and hyperventilation) in patients with PNES.21 It is possible that this phenomenon contributes to the shorter latency in our PNES patients; however, robust evidence that the course of PNES is affected by lowering doses in the EMU is lacking.
Limitations. This study has several limitations, including the retrospective design. The final diagnosis was obtained from discharge summaries and EMU reports but not verified by another source. In addition, risk factor profile was analyzed based on patient-reported symptoms that are subject to reporting bias. The AED taper was not uniform among all patients and influenced by provider preference. Chen-Block et al22 reported that patients with epilepsy/PNES (mixed) are more likely to require more than one EMU admission for definitive diagnosis. In that study, the first PNES event preceded an ES in 94.4 percent of patients with epilepsy/PNES. Thus, it is possible that those classified as PNES in our study could also have undiagnosed mixed epilepsy/PNES in the EMU evaluation. Additionally, by excluding the PNES/ES group, we discounted a group that has a reported prevalence rate of 7.3 or 32 percent of those with PNES.23
Conclusion
We conclude that in patients with high pre-EMU clinical suspicion for PNES, relatively shorter EMU monitoring (<48 hours) can capture clinical events and confirm diagnosis. This aids in early initiation of appropriate treatment and shortens length of stay. In addition, this phenomenon might improve cost-effectiveness of EMU monitoring in patients with PNES.
References
- Salinsky M, Spencer D, Boudreau E, Ferguson F. Psychogenic nonepileptic seizures in US veterans. Neurology. 2011;77:945–950.
- Seneviratne U, Reutens D, D’Souza W. Stereotypy of psychogenic nonepileptic seizures: insights from video-EEG monitoring. Epilepsia. 2010;51:1159–1168.
- Weaver DF. “Organic” pseudoseizures as an unrecognized side-effect of anticonvulsant therapy. Seizure. 2004;13:
467–469. - Hudak AM, Trivedi K, Harper CR, et al. Evaluation of seizure-like episodes in survivors of moderate and severe traumatic brain injury. J Head Trauma Rehabil. 2004;19:290–295.
- Kemp S, Graham CD, Chan R, et al. The frequency and management of seizures during psychological treatment among patients with psychogenic nonepileptic seizures and epilepsy. Epilepsia. 2018;59:844–853.
- LaFrance WC, Baker GA, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia. 2013;54:2005–2018.
- Friedman DE, Hirsch LJ. How long does it take to make an accurate diagnosis in an epilepsy monitoring unit? J Clin Neurophysiol. 2009;26(4):213–217.
- Cox FM, Reus EE, Visser GH. Timing of first event in inpatient long-term video-EEG monitoring for diagnostic purposes. Epilepsy Res. 2017;129:91–94.
- Perrin MW, Sahoo SK, Goodkin HP. Onset latency to a first psychogenic nonepileptic seizure during inpatient EEG monitoring: Evidence for semiological differences. Epilepsy & Behav. 2010;19(1):32–35.
- Foong M, Seneviratne U. Optimal duration of video-electroencephalographic monitoring to capture seizures. J Clin Neurosci. 2016;28: 55–60.
- Parra J, Kanner AM, Iriarte J, Gil-Nagel A. When should induction protocols be used in the diagnostic evaluation of patients with paroxysmal events? Epilepsia. 1998;39(8): 863–867.
- Lobello, K, Morgenlander JC, Radtke RA, Bushnell CD. Video/EEG monitoring in the evaluation of paroxysmal behavioral events: duration, effectiveness, and limitations. Epilepsy & Behav. 2006;8(1):261–266.
- Alper K, Devinsky O, Perrine K, et al. Nonepileptic seizures and childhood sexual and physical abuse. Neurology.
1993;43(10):1950–1953. - Ettinger AB, Devinsky O, Weisbrot DM, et al. Headaches and other pain symptoms among patients with psychogenic non-epileptic seizures. Seizure. 1999;8(7):424–426.
- Dworetzky BA, Strahonja-Packard A, Shanahan CW, et al. Characteristics of male veterans with psychogenic nonepileptic seizures. Epilepsia. 2005;46(9):1418–1422.
- D’Alessio L, Giagante B, Oddo S, et al. Psychiatric disorders in patients with psychogenic non-epileptic seizures, with and without comorbid epilepsy. Seizure. 2006;15(5):333–339.
- Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry.
1996;153(1):57–63. - Bautista RED, Gonzales-Salazar W, Ochoa JG. Expanding the theory of symptom modeling in patents with psychogenic nonepileptic seizures. Epilepsy & Behav. 2008;13:407–409.
- Woollacott IO, Scott C, Fish DR, et al. When do psychogenic nonepileptic seizures occur on a video/EEG telemetry unit? Epilepsy & Behav. 2010;17(2):228–235.
- Reuber M, Brown RJ. Understanding psychogenic nonepileptic seizures—phenomenology, semiology and the Integrative Cognitive Model. Seizure. 2017;44:199–205.
- Benbadis SR. Provocative techniques should be used for the diagnosis of psychogenic nonepileptic seizures. Epilepsy & Behav. 2009;15(2):106–109.
- Chen-Block S, Abou-Khalil BW, Arain A, et al. Video-EEG results and clinical characteristics in patients with psychogenic nonepileptic spells: The effect of a coexistent epilepsy. Epilepsy & Behav. 2016;62:62–65.
- El-Naggar H, Moloney P, Widdess-Walsh P, et al. Simultaneous occurrence of nonepileptic and epileptic seizures during a single period of in-patient video-electroencephalographic monitoring. Epilepsia. 2017;2(4):467–471.





