
by Zeeshan Mansuri, MD, MPH; Abhishek Reddy, MD; Ramu Vadukapuram, MD; Chintan Trivedi, MD, MPH; and Amy Amara, MD, PhD
Dr. Mansuri is with the Department of Psychiatry, Boston Children’s Hospital/Harvard Medical School in Boston, Massachusetts. Dr. Reddy is with the Department of Psychiatry, Virginia Tech Carilion School of Medicine in Roanoke, Virginia. Dr. Vadukapuram is with the Department of Psychiatry, Icahn School of Medicine at Mount Sinai in New York, New York. Dr. Trivedi is with St. David Medical Center in Austin, Texas. Dr. Amara is with the Department of Neurology at the University of Alabama at Birmingham in Birmingham, Alabama.
Funding: No funding was provided for this study.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Innov Clin Neurosci. 2022;19(1–3):46–51.
ABSTRACT: Background: Up to 60 percent of patients with Parkinson’s disease (PD) develop Parkinson’s disease psychosis (PDP). PDP is associated with a significant economic burden. The management of PDP has been divided into two approaches—one focuses on decreasing the doses of anti-Parkinsonian medications and the other involves prescribing atypical antipsychotics. Of these atypical antipsychotics, pimavanserin is United States (US) Food and Drug Administration (FDA)-approved specifically for the treatment of PDP.
Objective: The goal was to evaluate the safety and efficacy of pimavanserin in the treatment of PDP based on data from randomized clinical trials.
Methods: All the articles, which assessed pimavanserin’s effect on the treatment of PDP, were retrieved from Google Scholar, PubMed, and abstracts from annual scientific sessions. The data on dose, therapy duration, patient numbers, and study duration were collected. These data were analyzed with random effect modeling using the inverse variance method and the Mantel-Haenszel method.
Results: Four studies comparing pimavanserin to a placebo provided data on 680 patients (263 placebo, 417 pimavanserin). Treatment with pimavanserin was associated with a significant reduction in scores using the Scale of Assessment of Positive Symptoms, Hallucinations, and Delusion (SAPS-H+D) (mean difference [MD]: –1.55 [–2.71, –0.379], p=0.009). The groups had similar composite scores for Unified Parkinson’s Disease Rating Scale II and III (UPDRS II and III) (MD: 0.093 [–1.28, 1.46], p=0.89). Interestingly, pimavanserin was protective against orthostatic hypotension (risk ratio: 0.33 [0.30, 0.37], p<0.001). All other adverse events were similarly distributed across both groups.
Conclusion: There was a significant improvement in psychosis symptoms in patients with PD who took pimavanserin. Pimavanserin was also shown to be protective against orthostatic hypotension.
Keywords: Pimavanserin, Parkinson’s disease, psychosis
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that affects one percent of the population over the age of 60 years.1 The most significant risk factor is age, and the lifetime risk of PD in aging populations is expected to increase to 6.3 percent of women and 7.4 percent of men.2 The disease impacts over one million Americans annually, and the annual cost to the United States (US) economy is estimated to be $51.9 billion.3 Because there are no physiologic, radiologic, or blood tests that can confirm PD, diagnosis depends on clinical characteristics identified during one’s medical history and neurologic examination.4 Though we do not have any existing therapies to modify the underlying neurodegenerative process, symptomatic treatments can improve patient quality of life.5 Six chief classes of medications used for treating Parkinsonian motor symptoms are levodopa, dopamine agonists (DAs), monoamine oxidase type B (MAO B) inhibitors, catechol-O-methyltransferase (COMT) inhibitors, amantadine, and anticholinergic drugs.6 Adverse effects or complications of medication are the major challenges in the treatment of patients with PD. Medication-induced psychosis affects 59.5 percent of patients with PD. The risk of psychosis is increased in patients with older age at onset, higher doses of dopaminergic drugs, longer duration of disease, cognitive impairment, dementia, depression, and rapid eye movement (REM) sleep behavior disorder.7–9 Parkinson’s disease psychosis (PDP) is characterized by hallucinations, illusions, paranoia, and a false sense of presence. The severity of psychotic symptoms in PDP differs from that of primary psychotic disorders, such as schizophrenia. Auditory hallucinations (with command nature) or the presence of mania should alert the clinician to differentiate it from psychiatric cause. The majority of patients with PDP initially retain insight and clear sensorium, which are lost over time.10 The etiology of PDP is related to both the disease process and pharmacological management. While temporarily or permanently withdrawing or reducing the dose of dopaminergic medications might improve symptoms in patients with medication-induced psychosis, this strategy can also cause deterioration of clinical motor symptoms.11,12 The utility of the two antipsychotic medications that do not worsen Parkinsonism is limited because clozapine has a severe risk of agranulocytosis and quetiapine has not been found to be more effective than placebo.13 The affinity of antipsychotic drugs for dopamine D2 receptors varies significantly. Second-generation antipsychotics (SGAs) are generally thought to be safer in patients with PD due to their lower D2 antagonism, but they can also cause extrapyramidal symptoms, albeit at a lower rate than first-generation antipsychotics (FGAs).14 While Morgante et al15 observed that quetiapine and clozapine appeared to be equally effective in the treatment of PDP, Merims et al16 observed that clozapine was more effective at reducing the frequency of hallucinations and delusions, but it was associated with an increased risk of leukopenia. In a comparative study, Shotbolt et al17 summarized that four out of five placebo-controlled studies found quetiapine ineffective in PDP, whereas all of the head to head studies of quetiapine against clozapine and open-label studies found it to be effective.17–22
In April 2016, pimavanserin became the first US Food and Drug Administration (FDA)-approved drug for treating PDP.23 We performed a systematic review and meta-analysis of the safety and efficacy of pimavanserin in the treatment of PDP.
Methods
We retrieved data on PDP management from peer-reviewed, human-based, randomized, placebo-controlled clinical trials of pimavanserin published prior to June 2020. We retrieved information by searching PubMed, Google Scholar, clinicaltrials.gov, and abstracts from annual scientific sessions of neurology and psychiatry conferences. Search terms included “pimavanserin,” “Parkinson’s disease psychosis,” “Parkinson psychosis,” and “selective 5-hydroxy-tryptamine (5-HT2A) inverse agonist.” Only studies published in English, with a randomized design, placebo control groups, and human participants diagnosed with PDP were included. There were no filters set for age, sex, or number of participants. Reference lists from each article were examined to identify any other review articles and primary studies. After the final selection, we recorded efficacy outcome measures from the Scale for Assessment of Positive Symptoms, Hallucinations, and Delusion (SAPS-H+D) and Unified Parkinson’s Disease Rating Scale II and III (UPDRS II and III) composite scores, with higher scores indicating more severe motor symptoms. Additional collected data included the study year, medication dosage, duration of therapy, patient age, adverse events, and the number of patients leaving the studies due to adverse events.
Study selection. A total of 206 studies were screened as part of this meta-analysis. After eliminating review manuscripts, duplications, case reports, animal experiments, and nonrandomized studies, we kept two published randomized clinical trials24,25 and two randomized clinical trials listed on clinicaltrials.gov.26,27 In both published studies, there were two groups—pimavanserin and placebo. Both the clinical trials listed on clinicaltrials.gov had three groups to include different doses of pimavanserin. In one study,26 the three groups were pimavanserin 10mg, 20mg, and placebo, while the second studied pimavanserin 10mg, 40mg, and placebo.27
Statistical analysis. In the four studies included in this analysis, there were a total of six comparisons between pimavanserin and placebo. Because the two clinicaltrials.gov studies had two pimavanserin doses, we grouped the comparisons as Study A (pimavanserin 20mg vs. placebo) and Study B (pimavanserin 10mg vs. placebo) for one study.26 The comparison groups for the other study were Study C (pimavanserin 40mg vs. placebo) and Study D (pimavanserin 10mg vs. placebo).27 For Study B, efficacy data could not be retrieved, so it was not included in the efficacy analysis.
The heterogeneity across studies was evaluated with the Cochrane Q Chi-squared test. The inconsistency was assessed with the I2 test, which describes the percentage of the variability in effect estimates that is due to heterogeneity. Values of 25 percent, 50 percent, and 75 percent correspond to low, moderate, and high heterogeneity. For the efficacy analysis of SAPS-H+D score and UPDRS II and III composite score, we used each study’s least square mean difference and the subsequent 95% confidence interval (CI). The mean difference was calculated for summary effect.
To address the concern of moderate heterogeneity, we performed meta-regression and explored the covariates (mean age, year of study, dose, and therapy duration), which might have contributed to heterogeneity. The random effect method of DerSimonian and Laird was used for the analysis of SAPS-H+D score and UPDRS II and III composite score analysis.
The adverse event meta-analysis was performed for confusion, headache, hallucination, fall, edema, orthostatic hypotension, and discontinuation in the study due to side effects, using the random effect method of Mantel-Haenszel. In the adverse event analysis for the studies with three groups, both pimavanserin groups were combined into one group (i.e., data from Study B were included in Study A, and Study D data were included in Study C) to avoid a unit-of-analysis error. To assess the summary effects, forest plots were generated containing mean difference/relative risk and 95% CI. Publication bias was assessed using the Egger’s test and displayed as a funnel plot of precision. Mixed-effects meta-regression was used for meta-regression analysis. The regression coefficients of associated p-values were reported in the meta-regression analysis. An R2 coefficient was also calculated to indicate the percentage of variance explained by the covariates. The statistical analysis was performed with the Metafor and Meta packages,28,29 implemented in the R Foundation for Statistical Computing version 3.6.3.30 P-value of less than 0.05 was considered as statistically significant.
Results
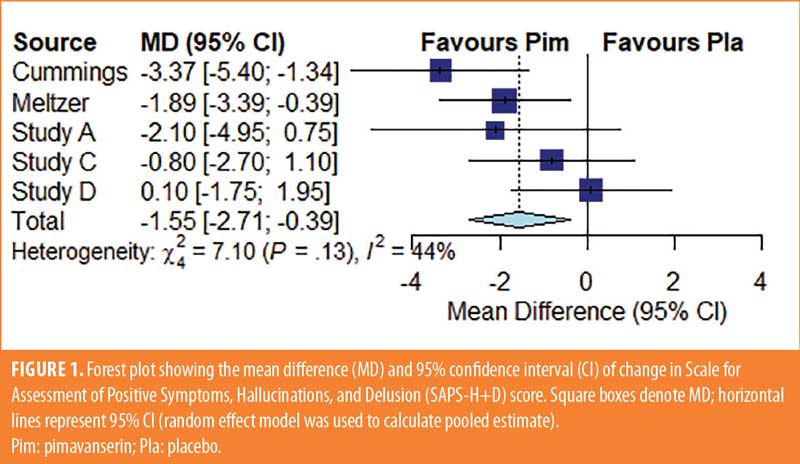
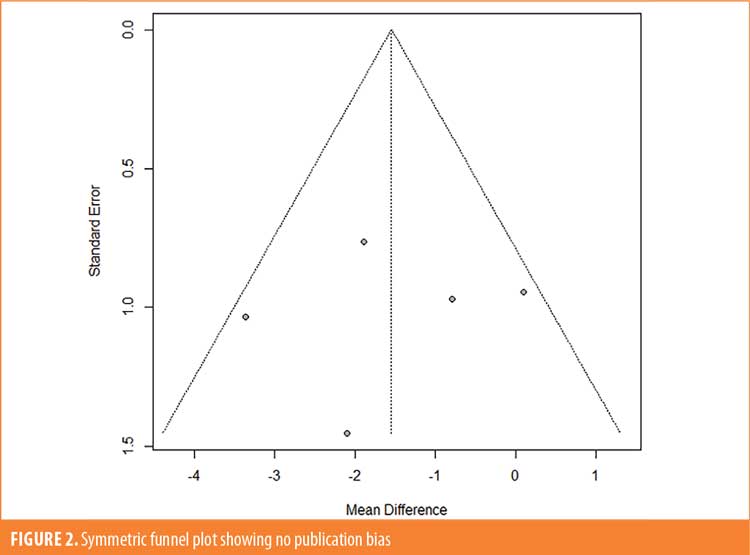
Study characteristics. Study characteristics are shown in Table 1, and patient characteristics for each study are included in Table 2. Cummings et al25 was a six-week study examining the impact of pimavanserin 40mg (95 patients) fixed dose/day compared to placebo (90 patients). The study by Meltzer et al24 was a four-week study with 29 participants receiving pimavanserin and 31 receiving a placebo. Patients were started at 20mg on Study Day 1, with possible increases to 40 or 60mg daily doses on Study Days 8 and 15, respectively, depending on their individual clinical responses. In the clinical trial, NCT00658567,26 123 participants received either a placebo (n=42), 10mg pimavanserin (n=41), or 20mg pimavanserin (n=40) every day for 6 weeks. In another six-week clinical trial, NCT00477672,27 98 patients were treated with a placebo, 101 patients with 10mg pimavanserin, and 99 patients with 40mg pimavanserin per day.
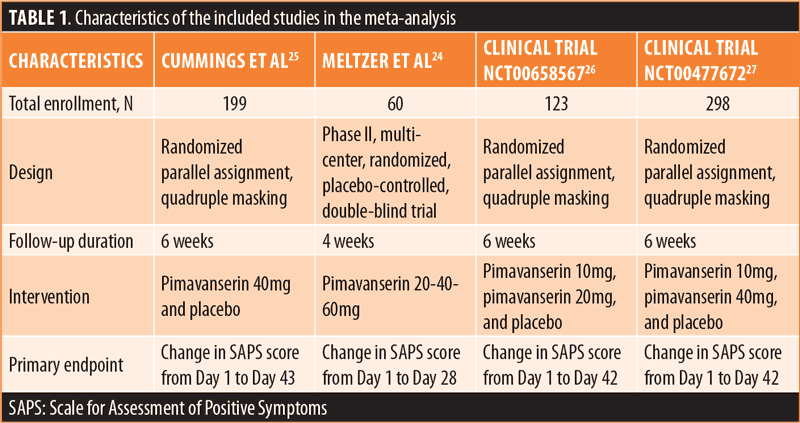
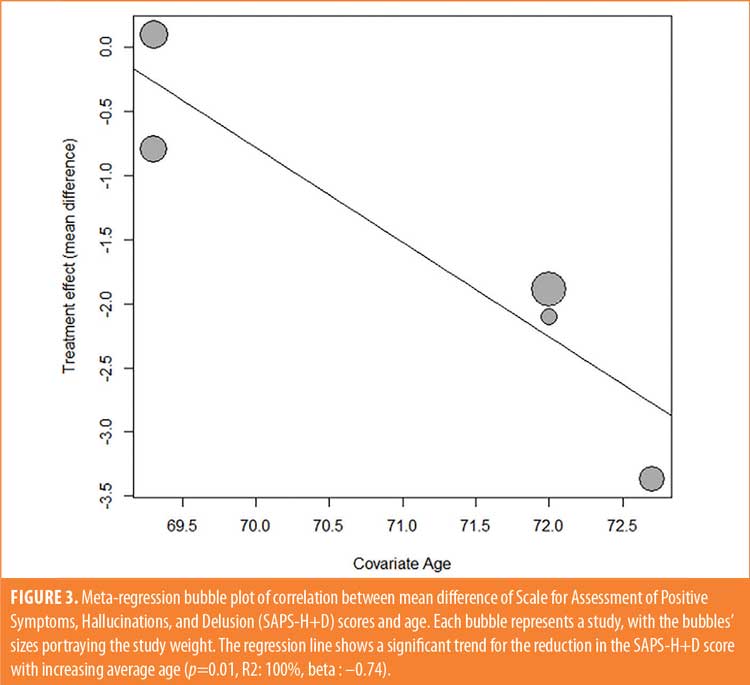
Efficacy outcomes. In the primary outcome analysis, treatment with pimavanserin was associated with a significant reduction in SAPS-H+D score (mean difference [MD]: –1.55 [–2.71, –0.379], p=0.009) (Figure 1). There was no publication bias on visual estimation (Figure 2) or by Egger’s test (p=0.82). There was a moderate heterogeneity of 44 percent. Meta-regression analysis investigated the potential effects of confounders on heterogeneity among the studies. In the meta-regression, we found that mean age was a significant predictor associated with the summary effect (beta: –0.74, p=0.01, R2: 100%) (Figure 3). This underlines that the mean SAPS-H+D score decreases more with an increase in mean age. There was no impact of years of the study (p=0.65) or four weeks versus six weeks of therapy (p=0.79) on the results. There was no statistically significant difference between the groups when considering the secondary outcome, the composite score for UPDRS II and III (MD: 0.093 [–1.28, 1.46], p=0.89) (Figure 4). There was no heterogeneity (I2: 0%) or publication bias (p=0.28)
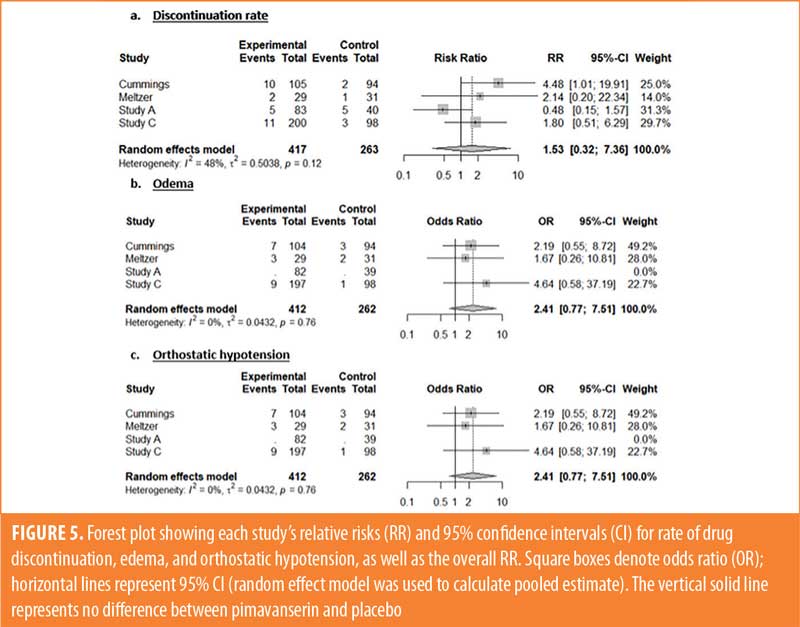
Safety outcomes. Orthostatic hypotension was less common in the pimavanserin group (risk ratio: 0.33 [0.30, 0.37], p<0.001) than it was in the placebo group. In addition, there was a notable slight trend toward significance for higher occurrence of edema in the pimavanserin group (risk ratio: 2.41, [0.77, –7.51], p=0.08). The groups had no difference in drug discontinuation rate (risk ratio: 1.53, [0.32, –7.36], p=0.97), confusion (p=0.37), headaches (p=0.58), hallucinations (p=0.22), and falls (p=0.60).

Discussion
Pimavanserin is a 5-HT2A inverse agonist and negative modulator of the 5-HT2A receptor approved for the treatment of psychosis in PD.31 To the best of our knowledge, this is the most updated meta-analysis and meta-regression of randomized clinical trials to evaluate the efficacy of pimavanserin on positive symptoms, associated with PD, such as hallucinations and delusions. This comprehensive meta-analysis also addresses the safety of pimavanserin when used to treat PDP.
The most significant finding in our study is the substantial reduction in hallucinations and delusions with pimavanserin, as shown by SAPS-H+D scores, compared to placebo in patients with PDP. Our meta-analysis also highlights the safety profile of pimavanserin, as it is associated with a decreased incidence of orthostatic hypotension when compared to the placebo group. It had a comparable safety profile to placebo for all other adverse effects, including confusion, headaches, and falls. Thus, we conclude that pimavanserin is safe and effective when used in the treatment of PDP.
Ours is not the first meta-analysis on this topic. There was a meta-analysis by Yasue et al,32 which also showed the efficacy of pimavanserin in PDP; however, all the groups we studied were not included in that study. Also, there was another meta-analysis on the effect of atypical antipsychotics on PDP, in which the authors concluded that pimavanserin and clozapine were both effective. However, clozapine is associated with severe side effects.33 In that study, the authors performed a subanalysis of pimavanserin for efficacy outcome using data from the same studies we included; however, we noticed a discrepancy in the MD values, and safety analysis was not performed. In a recently published study on the safety and efficacy of atypical antipsychotics for the treatment of PDP, pimavanserin showed a favorable profile, but efficacy was inferior to clozapine.34 In that study, only two published clinical trials were included. None of the meta-analyses32–34 evaluated other factors associated with the pooled efficacy outcome by meta-regression. In our study, we performed meta-regression and noticed that reduction in the SAPS-H+D score was greater with an increase in age.
Pimavanserin, like other antipsychotic medications, can cause QT interval prolongation (mean increase in QTc 5–8ms with a dose of 34mg/d). As a result, it should be avoided in patients who are taking other medications that prolong the QT interval, such as antipsychotics, and in those patients with known QT prolongation or history of cardiac arrhythmias.35,36 In Cummings et al,25 mean increase of 73ms in QT correction with Bazett formula (QTcB) interval was reported with pimavanserin from baseline to Day 43, compared to no change with placebo. In the pimavanserin group, there was a small but noticeable increase in QT interval that was not associated with cardiac adverse events.25 Pimavanserin, like other classes of antipsychotic medications, carries a black box warning about an increased risk of death in elderly patients with dementia.25
Limitations. Despite the strengths of this study in compiling available evidence on the efficacy and safety of pimavanserin for the treatment of PDP, there are also several limitations to consider. First, few trials were available for inclusion in the meta-analysis, and the available trials had relatively small sample sizes. In addition, the dose of pimavanserin was different between the included studies, making comparisons more complex. Furthermore, there was high heterogeneity. While age was one identified source of heterogeneity, there might be other sources that were not captured in this analysis. Also, all the trials were sponsored by Acadia Pharmaceuticals; there were no independent trials on the drug, which is a limiting factor. Industry sponsorship might potentially result in bias that can impact research at various stages. Information collected from a variety of fields have revealed biases in research design, conduct, and publication that are related to industry funding sources.37,38 Industry sponsorship might also have an impact on the research agenda when the research questions are chosen and framed, which is the first step in conducting research. Bias in the research agenda can lead to results that support only certain policy responses to pressing public health problems, affecting the health of the population.39 Finally, our results are limited to only the SAPS-H+D scale because of limited data; however, there are other scales that can help assess the efficacy of pimavanserin.
Conclusion
Pimavanserin appears beneficial in the treatment of PDP, is well-tolerated compared to placebo, and did not show significant safety concerns. This study could set the stage for further research with larger prospective, randomized, controlled trials that would explore pimavanserin’s role in not just the treatment of PDP, but also other types of psychosis in patients without PD. Additional studies can also incorporate long-term outcomes, the ideal duration of treatment, and comparisons with other treatment modalities, including clozapine and quetiapine.
Author Contributions
Dr. Mansuri and Dr. Reddy share equal credits for the first authorship.
References
- Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. Journal of neural transmission. J Neural Transm (Vienna). 2017;124(8):901–905.
- Wanneveich M, Moisan F, Jacqmin-Gadda H, et al. Projections of prevalence, lifetime risk, and life expectancy of Parkinson’s disease (2010-2030) in France. Mov Disord. 2018;33(9):1449–1455.
- The Michael J. Fox Foundation for Parkinson’s Research. Economic burden and future impact of Parkinson’s disease: final report. 5 Jul 2019. https://www.michaeljfox.org/sites/default/files/media/document/2019%20Parkinson%27s%20Economic%20Burden%20Study%20-%20FINAL.pdf. Accessed 20 Jan 2022.
- Tarakad A, Jankovic J. Diagnosis and management of Parkinson’s disease. Semin Neurol. 2017;37(2):118–126.
- Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670–1683.
- Fox SH, Katzenschlager R, Lim SY, et al. International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33(8):1248–1266.
- Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson disease. Arch Neurol. 2010;67(8):996–1001.
- Sanchez-Ramos JR, Ortoll R, Paulson GW. Visual hallucinations associated with Parkinson disease. Arch Neurol. 1996;53(12):1265–1268.
- Aarsland D, Larsen JP, Cummins JL, Laake K. Prevalence and clinical correlates of psychotic symptoms in Parkinson disease: a community-based study. Arch Neurol. 1999;56(5):595–601.
- Stacy M, Jakel R. Parkinson’s disease psychosis. J Parkinsonism Restless Legs Syndrome. 2014;4:41–51.
- Friedman JH. ’Drug holidays’ in the treatment of Parkinson’s disease. A brief review. Arch Intern Med. 1985;145(5):913–915.
- Friedman JH. Parkinson disease psychosis: update. Behav Neurol. 2013;27(4):469–477.
- Chen S, Chan P, Sun S, et al. The recommendations of Chinese Parkinson’s disease and movement disorder society consensus on therapeutic management of Parkinson’s disease. Transl Neurodegener. 2016;5:12.
- Divac N, Prostran M, Jakovcevski I, Cerovac N. Second-generation antipsychotics and extrapyramidal adverse effects. Biomed Res Int. 2014;2014:656370.
- Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in Parkinsonian patients with dopaminergic psychosis. Clin Neuropharmacol. 2004;27(4):153–156.
- Merims D, Balas M, Peretz C, et al. Rater-blinded, prospective comparison: quetiapine versus clozapine for Parkinson’s disease psychosis. Clin Neuropharmacol. 2006;29(6):331–337.
- Shotbolt P, Samuel M, David A. Quetiapine in the treatment of psychosis in Parkinson’s disease. Ther Adv Neurol Disord. 2010;3(6):339–350.
- Ondo WG, Tintner R, Voung KD, et al. Double-blind, placebo-controlled, unforced titration parallel trial of quetiapine for dopaminergic-induced hallucinations in Parkinson’s disease. Mov Disord. 2005;20(8):958–963.
- Rabey JM, Prokhorov T, Miniovitz A, et al. Effect of quetiapine in psychotic Parkinson’s disease patients: a double-blind labeled study of 3 months’ duration. Mov Disord. 2007;22(3):313–318.
- Kurlan R, Cummings J, Raman R, et al. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology. 2007;68(17):1356–1363.
- Shotbolt P, Samuel M, Fox C, David AS. A randomized controlled trial of quetiapine for psychosis in Parkinson’s disease. Neuropsychiatr Dis Treat. 2009;5:327–332.
- Fernandez HH, Okun MS, Rodriguez RL, et al. Quetiapine improves visual hallucinations in Parkinson disease but not through normalization of sleep architecture: results from a double-blind clinical-polysomnography study. Int J Neurosci. 2009;119(12):2196–2205.
- United States Food and Drug Administration. FDA approves first drug to treat hallucinations and delusions associated with Parkinson’s disease. 1 Mar 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treat-hallucinations-and-delusions-associated-parkinsons-disease. Accessed 20 Jan 2022.
- Meltzer HY, Mills R, Revell S, et al. Pimavanserin, a serotonin(2A) receptor inverse agonist, for the treatment of parkinson’s disease psychosis. Neuropsychopharmacology. 2010;35(4):881–892.
- Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533–540.
- ClinicalTrials.gov. A study of safety and efficacy of pimavanserin (ACP-103) in patients with Parkinson’s disease psychosis. https://clinicaltrials.gov/ct2/show/NCT00658567. Accessed 20 Jan 2022.
- ClinicalTrials.gov. A study of the safety and efficacy of pimavanserin (ACP-103) in patients with Parkinson’s disease psychosis. https://clinicaltrials.gov/ct2/show/NCT00477672. Accessed 20 Jan 2022.
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Statistical Software. 2010;36(3):1-48.
- Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160.
- The R Foundation. R: The R Project for Statistical Computing. https://www.r-project.org. Accessed 20 Jan 2022.
- Lyons KE, Pahwa R, Hermanowicz N, et al. Changing the treatment paradigm for Parkinson’s disease psychosis with pimavanserin. Expert Rev Clin Pharmacol. 2019:12(7):681–691.
- Yasue I, Matsunaga S, Kishi T, et al. Serotonin 2A receptor inverse agonist as a treatment for Parkinson’s disease psychosis: a systematic review and meta-analysis of serotonin 2A receptor negative modulators. J Alzheimers Dis. 2016;50(3):733–740.
- Zhang H, Wang L, Fan Y, et al. Atypical antipsychotics for Parkinson’s disease psychosis: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2019;15:2137–2149.
- Iketani R, Furushima D, Imai S, Yamada H. Efficacy and safety of atypical antipsychotics for psychosis in Parkinson’s disease: A systematic review and Bayesian network meta-analysis. Parkinsonism Relat Disord. 2020;78:82–90.
- Ballard C, Isaacson S, Mills R, et al. Impact of current antipsychotic medications on comparative mortality and adverse events in people with Parkinson disease psychosis. J Am Med Dir Assoc. 2015;16(10):898.e1–898.e8987.
- Hawkins T, Berman BD. Pimavanserin: a novel therapeutic option for Parkinson disease psychosis. Neurol Clin Pract. 2017;7(2):157–162.
- Lundh A, Lexchin J, Mintzes B, et al. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2017;2(2):MR000033.
- Bero L, Oostvogel F, Bacchetti P, Lee K. Factors associated with findings of published trials of drug-drug comparisons: why some statins appear more efficacious than others. PLoS Med. 2007;4(6):e184.
- Fabbri A, Lai A, Grundy Q, Bero LA. The influence of industry sponsorship on the research agenda: a scoping review. Am J Public Health. 2018;108(11):e9–e16.





