 by Seth C. Hopkins, PhD; Ajay Ogirala, PhD, Antony Loebel, MD; and Kenneth S. Koblan, PhD
by Seth C. Hopkins, PhD; Ajay Ogirala, PhD, Antony Loebel, MD; and Kenneth S. Koblan, PhD
Drs. Hopkins, Ogirala, Loebel, and Koblan are with Sunovion Pharmaceuticals Inc, Marlborough, Massachusetts.
Funding: Funding was provided by Sunovion Pharmaceuticals Inc.
Disclosures: The authors are employees of Sunovion Pharmaceuticals Inc.
Abtract: The Positive and Negative Syndrome Scale (PANSS) is the most widely used efficacy measure in acute treatment studies of schizophrenia. However, interpretation of the efficacy of antipsychotics in improving specific symptom domains is confounded by moderate-to-high correlations among standard (Marder) PANSS factors. The authors review the results of an uncorrelated PANSS score matrix (UPSM) transform designed to reduce pseudospecificity in assessment of symptom change in patients with schizophrenia. Based on a factor analysis of five pooled, placebo-controlled lurasidone clinical trials (N=1,710 patients), a UPSM transform was identified that generated PANSS factors with high face validity (good correlation with standard Marder PANSS factors), and high specificity/ orthogonality (low levels of between-factor correlation measuring change during treatment). Between-factor correlations were low at baseline for both standard (Marder) PANSS factors and transformed PANSS factors. However, when measured change in symptom severity was measured during treatment (in a pooled 5-study analysis), there was a notable difference for standard PANSS factors, where changes across factors were found to be highly correlated (factors exhibited pseudospecificity), compared to transformed PANSS factors, where factor change scores exhibited the same low levels of between-factor correlation observed at baseline. At Week 6-endpoint, correlations among PANSS factor severity scores were moderate-to-high for standard factors (0.34–0.68), but continued to be low for the transformed factors (-0.22–0.20). As an additional validity check, we analyzed data from one of the original five pooled clinical trials that included other well-validated assessment scales (MADRS, Negative Symptom Assessment scale [NSA]). In this baseline analysis, UPSM-transformed PANSS factor severity scores (negative and depression factors) were found to correlate well with the MADRS and NSA. The availability of transformed PANSS factors with a high degree of orthogonality/specificity, but which retain a high degree of concurrent and face validity, can reduce pseudospecificity as a measurement confound, and should facilitate the drug development process, permitting a more accurate characterization of the efficacy of putative new agents in targeting specific symptom domains in patients with psychotic illness.
Keywords: Schizophrenia, antipsychotic agents, factor analysis, efficacy, clinical, clinical trials
Innov Clin Neurosci. 2017;14(11–12):54–58.
Introduction
Schizophrenia is a chronic remitting and relapsing disorder characterized by a wide range of psychological, behavioral and cognitive symptoms. Over more than half a century, dozens of antipsychotics have received regulatory approval, in the United States and internationally, for the treatment of schizophrenia. In 1987, Kay, Fishbein, and Opler1 introduced the Positive and Negative Syndrome Scale (PANSS) as an “operationalized, drug-sensitive instrument that provides balanced representation of positive and negative symptoms and gauges their relationship to one another and to global psychopathology”. Since its introduction, the PANSS has been the most widely used measure of efficacy in clinical trials in patients with schizophrenia. The original validation article has been cited in more than 14,000 published articles.
The PANSS was developed by combining items from the Brief Psychiatric Rating Scale2 and the Psychopathology Rating Schedule,3 and enhancing the psychometric properties of the resulting instrument by clearly operationalizing item definitions and severity anchor points. The aim was to provide a reliable and valid measure of the two-factor (positive and negative) classification of schizophrenic symptoms that had been proposed by Crow in 1980.4 The reliability and validity of the PANSS as a measure of outcome was demonstrated for the original positive, negative, and general psychopathology subscales.5
In the decade after its introduction, it became clear that the two-factor model of psychopathology (or three-factor, including general psychopathology) was not optimal to fully characterize key symptom dimensions of schizophrenia or outcome in response to treatment. Factor and principal component analyses of the PANSS have consistently identified five factors that map to the diagnostic criteria of positive symptoms, negative symptoms, disorganized thinking, and the associated symptoms of hostility/excitement and depression/anxiety.6–10 For the past two decades, the PANSS total score and the five so-called “Marder Factor” scores have been standard metrics for assessing efficacy in schizophrenia clinical trials.
PANSS Factors and Assessment of Efficacy: The Challenge of Pseudospecificity
For the standard (Marder) PANSS factors at baseline, the shared variance between positive and negative symptom severity scores is relatively low, indicating a degree of separation in the underlying neurobiological substrate.6–9 This finding is consistent with evidence that the neurobiological correlates of positive symptoms are largely different from those that underlie negative symptoms.11–13
In contrast, the standard (Marder) PANSS factor change scores have been found to be highly correlated with each other. Especially notable is the high degree of correlation between the PANSS positive factor and other Marder factors. We have confirmed this finding in a previous pooled analysis of PANSS data derived from five double-blind, placebo-controlled studies of lurasidone for the treatment of patients with an acute exacerbation of schizophrenia.14 Moderate-to-high correlations were observed between improvements in standard (Marder) PANSS positive and other PANSS factors, ranging from r=0.52 to r=0.74. Correlations of this magnitude represent a major confound that make interpretation of treatment-related improvement in individual Marder factors challenging. As a result, improvement in symptom severity in one of the Marder factors might not represent a treatment effect on a specific symptom domain, but instead might simply be a nonspecific effect secondary to improvement in PANSS items that are highly correlated with the PANSS positive factor.
The high degree of between-factor correlation among Marder PANSS factors has been characterized as an example of pseudospecificity. Pseudospecificity is the term, originally promulgated by the United States Food and Drug Administration (FDA),15,16 to describe potential pharmacologic symptom targets that are too highly correlated (overlapping) in terms of phenomenology and measured treatment response to justify separate drug treatment claims.
Possible Solutions to the Problem of Pseudospecificity
There are two possible approaches that can be used to address the measurement issue that is central to pseudospecificity, both of which have advantages. One approach is to direct research efforts toward the development and validation of new, domain-specific, instruments with minimal-to-no correlation with other outcome domains. Examples of this approach include instruments such as the Negative Symptom Assessment (NSA) Scale17–19 and the MATRICS Consensus Cognitive Battery (which measures cognitive function).20,21 However, to our knowledge, evidence has not been presented demonstrating that instruments (such as the NSA) have low levels of correlation with PANSS positive (and other) factors in patients experiencing an acute exacerbation of schizophrenia.
A second approach would retain the PANSS as an efficacy measure while using analytic strategies to minimize the degree of between-factor correlation. The advantage of this approach is that it is a cost-effective strategy that would permit acquisition of domain-specific efficacy data using required acute registration trials without the need to conduct, for example, a separate negative symptom trial. In addition, validation of modified PANSS factors with low between-factor correlation would facilitate study replication while preserving decades of treatment research with dozens of agents, many with distinctive receptor binding profiles, that could be retrospectively analyzed for domain-specific efficacy.
We briefly summarize here recently reported results of an uncorrelated PANSS score matrix (UPSM) transform designed to reduce pseudospecificity in the assessment of symptom change in patients with schizophrenia.14 We also report new results that assess whether the UPSM transform can also be applied directly to PANSS severity scores at baseline.
Transformed PANSS factors: High Correlation with Standard (Marder) Factors
The original endpoint change score analysis14 was performed on PANSS data derived from five similarly designed, randomized, double-blind, placebo-controlled, six-week treatment studies of lurasidone or active comparator for the treatment of patients (N=1,710) with an acute exacerbation of schizophrenia. In this analysis, each of the seven transformed PANSS factors was found to have a moderate-to-high degree of correlation with its respective standard (Marder) factor: for the positive factor (0.79), for the disorganized factor (0.79), for negative-apathy/avolition with negative symptoms (0.75), for negative-deficit of expression with negative symptoms (0.65), for hostility/excitement (0.94), for anxiety with anxiety/depression (0.74), and for depression with anxiety/depression (0.76). These levels of correlation suggest that the transformed PANSS factors are measuring the same symptom domains (constructs) as the Marder factors.
Transformed PANSS factors: Low Between-factor Correlation
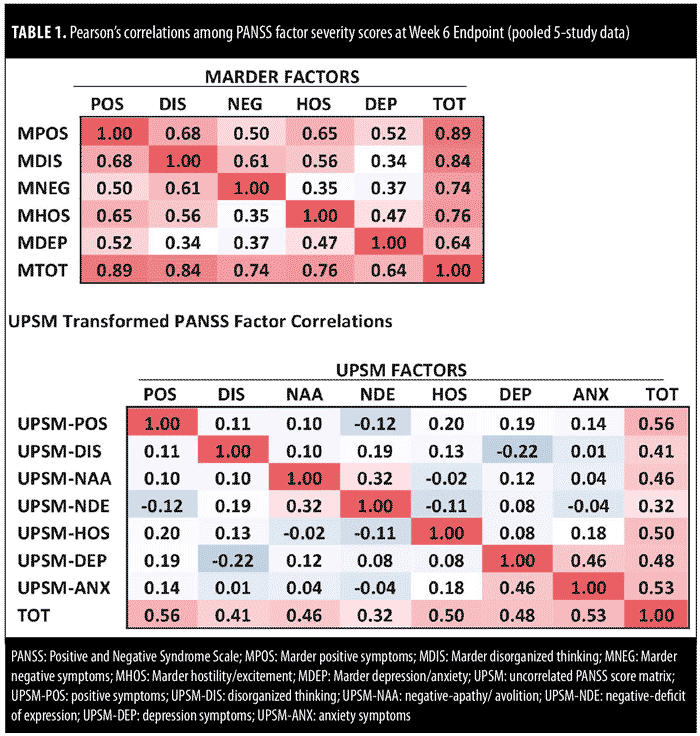
In our original endpoint change score analysis of five pooled clinical trials,14 transformed PANSS factors exhibited markedly reduced between-factor correlations when compared to the between-factor correlations observed for the standard (Marder) PANSS factors. Pearson’s between-factor correlations ranged from 0.40 to 0.74 for endpoint change in the standard (Marder) PANSS factors, and from -0.01 to 0.27 in the transformed PANSS factors (with 0.27 being the correlation between the anxiety and depression subfactors). The low levels of between-factor correlation among the transformed PANSS factors suggest that these reconfigured factors are orthogonal to each other and are successfully measuring the effect of treatment with a high degree of specificity.
Transformed PANSS Factors: Lurasidone vs. Placebo Effect Sizes
In an additional analysis of the five pooled clinical trials,14 effect size estimates for endpoint change (lurasidone vs. placebo) were calculated using both standard (Marder) PANSS factors, and transformed PANSS factors. Effect size estimates exhibited a relatively consistent pattern across the standard (Marder) factors, ranging from 0.31 to 0.44. In contrast, greater between-factor heterogeneity was observed in effect size estimates using the transformed PANSS factors, ranging from 0.05 to 0.27, with greater lurasidone effects observed on positive and hostility symptoms, and smaller drug effects on disorganized, negative apathy/avolition, deficit of expression, and anxiety/depression symptoms.
Transformed PANSS factors: Cross-study Validation
In a validation analysis using 12 separate clinical trials,14 we confirmed that the weighted UPSM coefficients had generalizable utility, yielding transformed PANSS factors with high specificity while retaining good levels of correlation with standard PANSS factors.
Application of UPSM transform to PANSS Severity Scores at Baseline
In an analysis applying the UPSM PANSS factor transform to pooled baseline five study data, UPSM transformed PANSS factor scores at baseline were found to correlate well with their respective standard (Marder) PANSS factor scores at baseline, with Pearson’s correlations of transformed versus standard PANSS factors in the moderate-to-high range for PANSS positive (0.53), disorganized (0.84), negative symptoms, apathy/avolition (0.74), negative symptoms, deficit of expression (0.76), hostility (0.95), anxiety (0.75), and depression (0.84).
Between-factor correlations for both standard (Marder) PANSS factors and transformed PANSS factors were low at baseline. For example, the correlation between Marder PANSS positive and negative factors was -0.03 at baseline. Inverse correlations were noted for transformed PANSS positive and negative factors (-0.18 for negative-apathy/avolition; -0.26 for negative-deficit of expression).
The concurrent validity of selected standard and transformed PANSS factor severity scores at baseline was assessed based on Pearson’s correlations. The correlation at baseline between MADRS total score and standard (Marder) PANSS depression/anxiety factor and transformed PANSS depression factor was 0.56 and 0.53, respectively. The correlation at baseline between the NSA scale score and standard PANSS negative symptom score was 0.68, and the correlation with the NSA score was 0.53 for the transformed PANSS negative-apathy/avolition subscore and 0.55 for transformed PANSS negative-deficit of expression subscore.
Structure of (Baseline) Symptom Severity vs. Structure of Symptom change
As summarized above, baseline severity scores exhibit low levels of between-factor correlation using both standard (Marder) and transformed PANSS factors. However, when measuring change in symptom severity during treatment, there is a notable difference between standard PANSS factors, where change across factors is found to be highly correlated (factors exhibit pseudospecificity), and transformed PANSS factors, which continue to have the same low levels of between-factor correlation observed at baseline (i.e., factors may be characterized as exhibiting ergodicity).
Table 1 summarizes, in an analysis of pooled five-study data, Pearson’s correlations between PANSS severity scores at Week 6-endpoint, which provide additional confirmation of this difference. In the top panel of Table 1, correlations among standard (Marder) PANSS factors at Week 6 range from 0.34 to 0.68. In contrast, correlations among transformed PANSS factors is notably lower (-0.22–0.20, excluding correlations between negative subscores and depression and anxiety subscores).
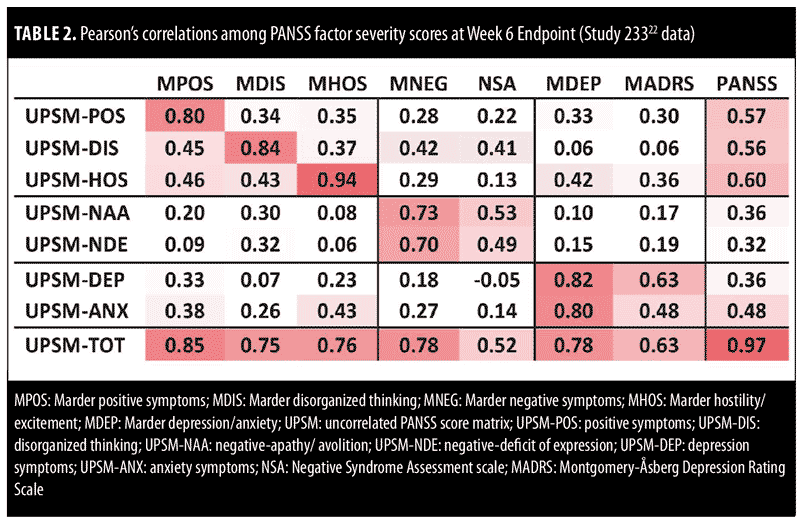
In an analysis of Week 6 data from Study 233 (1 of the 5-pooled studies),22 transformed PANSS factor severity scores showed high levels of correlation with the respective standard (Marder) PANSS factors, with correlations ranging from 0.73 to 0.94 (Table 2). Transformed PANSS factor severity scores at Week 6 also showed moderate levels of correlation between the NSA total score and the negative-apathy/avolition subscore (0.53) and the negative deficit of expression subscore (0.49). Similarly, the transformed PANSS factor depression severity score showed moderate levels of correlation with the MADRS total score (0.63, Table 2).
Perspective
We have briefly summarized here a factor analytic procedure that generated weighted coefficients which, when applied to each of the 30 PANSS items utilized in schizophrenia clinical trials, yields transformed PANSS factors that meet four important criteria: 1) they have high face validity (i.e., they exhibit medium-to-large correlations with the standard [Marder] PANSS factors, and thus appear to be measuring the same clinical symptom domains in schizophrenia; 2) they have high orthogonality, and, thus, because of the low level of between-factor correlation, they are measuring the effect of a drug on a symptom domain with a high degree of specificity; 3) they exhibit minimal loss of information (i.e., after the UPSM transformation, >90% of information variance was retained, with high R-squared values between sums of the transformed PANSS factors and PANSS total score); and 4) the weighted UPSM coefficients have generalizable utility, and, thus, can be applied to PANSS data in a wide range of clinical trials to generate transformed PANSS factors with the same clinimetric properties as the original transformed PANSS factors.
Effect size estimates for endpoint change (lurasidone vs. placebo) were notably different when calculated using the standard (Marder) PANSS factors and the transformed PANSS factors. The uniformity of effect sizes calculated using the Marder PANSS factors reflects the high level of between-factor correlation, particularly for the PANSS positive factor (range: r, 0.52–0.74). This level of between-factor correlation contributes to the likely overestimate of the effect of treatment on many of the PANSS factors. The effect size profile of lurasidone, when estimated using transformed PANSS factors, would appear to provide a more valid measure of the efficacy of lurasidone in treating the key clinical symptoms domains of schizophrenia. An important next step will be to replicate these Results for other antipsychotic agents, both first and second generation.
The results of the UPSM analysis of baseline and Week 6 (on treatment) data confirm and extend our previously reported14 UPSM change score analysis. At baseline, standard (Marder) PANSS factor severity scores and transformed PANSS factor severity scores both exhibited low levels of between-factor correlation, suggesting that each factor is measuring a separate clinical symptom domain. However, when measuring the effect of treatment, standard (Marder) PANSS factor change scores exhibited moderate between-factor correlations, indicative of pseudospecificity, while transformed PANSS changes scores exhibited ergodicity, since they continue to show the same domain-independent factor structure that was observed at baseline. Thus, the UPSM transform yields results that support the notion that there is a consistent underlying schizophrenia symptom structure present in both exacerbated and stable (treated) states.
Previously,we showed that the transformed PANSS factor change scores were highly correlated with their respective standard (Marder) PANSS factor change scores,14 indicating that the transformed PANSS factors are measuring the same symptom domains as the Marder factors. In the new analyses reported here, we have shown that transformed PANSS factor scores were highly correlated with their respective standard (Marder) PANSS factor scores at baseline. In addition, we have summarized analyses from a single study where MADRS and NSA assessments were available that provide preliminary evidence of concurrent validity between these measures and the transformed PANSS depression and negative symptom subscores, respectively.
Clinical Implications
For more than a quarter of a century, the PANSS has been the gold standard measure of efficacy for clinical trials in schizophrenia. However, the high degree of correlation among Marder PANSS factors has proved to be its Achilles heel, preventing clinicians, researchers, and regulatory agencies alike from clearly determining whether improvement in (for example) the PANSS negative factor is a targeted drug effect or whether (and to what extent) negative symptom improvement might reflect improvement in a (correlated) PANSS factors.
The term pseudospecificity was coined to describe improvement in a symptom domain that is highly correlated (overlapping) with a separate outcome domain in terms of phenomenology and measured treatment response. We view the current UPSM procedure for generating transformed PANSS factors as a means to generate reliable signals of drug effect across symptom domains in acute treatment trials of patients with schizophrenia. In an acute trial involving exacerbated patients, demonstration of improvement in, for example, the transformed negative symptom factor might be viewed as a more reliable indicator of specific treatment effect than change in the standard PANSS negative symptom subscale. Such signal-generating results may then be used to support confirmatory studies or could, in some cases, be potentially used for labelling without the need for additional studies.
In addition, the availability of transformed PANSS factors with a high degree of orthogonality/specificity should help clinicians to have a better understanding of the structure of symptom change in schizophrenia and the profile of treatment effects across schizophrenia symptom domains. Thus, reanalysis of existing clinical trials data sets using transformed PANSS factors could provide important insights into the effectiveness of existing antipsychotic agents for the treatment of specific symptom domains in patients with schizophrenia.
Conclusion
Looking forward, the availability of transformed PANSS factors should provide a more valid method for measuring domain-specific effects of candidate antipsychotics, especially candidate drugs with non-D2 receptor binding mechanisms of action. It is hoped that the availability of valid measures of symptom change, without the confound of pseudospecificity, might facilitate the drug development process, permitting a more accurate characterization of the efficacy of putative new agents in targeting specific symptom domains in patients with psychotic illness.
References
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13: 261–276.
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:790–812.
- Singh MM, Kay SR. A comprehensive study of haloperidol and chlorpromazine in terms of clinical effects and therapeutic reversal with benztropine in schizophrenia: theoretical implications for potency differences among neuroleptics. Psychopharmacologia. 1975;43:115–123.
- Crow T. Molecular pathology of schizophrenia: more than one dimension of pathology? BMJ. 1980;280:66–68.
- Kay SR, Opler LA, Fiszbein A. The Positive and Negative Syndrome Scale (PANSS) Manual. Toronto, ON: Multi-Health Systems Inc.; 2000.
- Lindenmayer JP, Bernstein-Hyman R, Grochowski S, et al. Psychopathology of schizophrenia: initial validation of a five-factor model. Psychopathology. 1995;28:22–3.
- Lindenmayer JP, Grochowski S, Hyman RB. Five factor model of schizophrenia: replication across samples. Schizophr Res. 1995;14:229–234.
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58:538–546.
- Emsley R, Rabinowitz J, Torreman M, Group R-I-EPGW. The factor structure for the Positive and Negative Syndrome Scale (PANSS) in recent-onset psychosis. Schizophr Res. 2003;61:47–57.
- Wallwork RS, Fortgang R, Hashimoto R, et al. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137:246–250.
- Hopkins SC, Ogirala A, Loebel A, Koblan KS. Transformed PANSS factors intended to reduce pseudospecificity among symptom domains and enhance understanding of symptom change in antipsychotic-treated patients with schizophrenia. Schizophr Bull 2017 Sep 11. doi: 10.1093/schbul/sbx101. [Epub ahead of print].
- Galderisi S, Merlotti E, Mucci A. Neurobiological background of negative symptoms. Eur Arch Psychiatry Clin Neurosci. 2015;265:543–58
- Gruber O, Santuccione AC, Aach H. Magnetic resonance imaging in studying schizophrenia, negative symptoms, and the glutamate system. Front Psychiatry. 2014;5:32.
- Zhu J, Zhuo C, Liu F, et al. Neural substrates underlying delusions in schizophrenia. Sci Rep. 2016;6:33857.
- Leber P. Regulatory issues, p.485-494. In: Kenneth L. Davis DC, Joseph T. Coyle, and, Nemeroff C, eds. Neuropsychopharmacology: The Fifth Generation of Progress: Lippincott, Williams, & Wilkins, Philadelphia, PA, 2002.
- Laughren T, Levin T. Food and Drug Administration perspective on negative symptoms in schizophrenia as a target for a drug treatment claim. Schizophr Bull. 2006;32:220-222.
- Raskin A, Pelchat R, Sood R, et al. Negative symptom assessment of chronic schizophrenia patients. Schizophr Bull. 1993;19:627-35.
- Axelrod BN, Goldman RS, Woodard JL, Alphs LD. Factor structure of the negative symptom assessment. Psychiatry Res. 1994;52:173–9.
- Garcia-Portilla MP, Garcia-Alvarez L, Saiz PA, et al. Psychometric evaluation of the negative syndrome of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2015;265:559–566.
- Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213.
- Keefe RS, Haig GM, Marder SR, et al. Report on ISCTM consensus meeting on clinical assessment of response to treatment of cognitive impairment in schizophrenia. Schizophr Bull. 2016;42:19–33.
- Loebel A, Cucchiaro J, Sarma K, et al. Efficacy and safety of lurasidone 80mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res. 2013;145:101–9.





