 by Ibrahim Turkoz, PhD; Dong-Jing Fu, MD, PhD; Cynthia A. Bossie, PhD; Larry Alphs, MD, PhD
by Ibrahim Turkoz, PhD; Dong-Jing Fu, MD, PhD; Cynthia A. Bossie, PhD; Larry Alphs, MD, PhD
Dr. Turkoz is with Janssen Research and Development LLC, Titusville, New Jersey, USA, and Drs. Fu, Bossie, and Alphs are with Janssen Scientific Affairs LLC, Titusville, New Jersey, USA.
Innov Clin Neurosci. 2015;12(11–12):10–17.
Funding: Funding for this study was provided by Janssen Scientific Affairs, LLC.
Financial disclosures: Dr. Turkoz is an employee of Janssen Research and Development LLC and a Johnson & Johnson stockholder; Drs. Fu, Bossie, and Alphs are employees of Janssen Scientific Affairs LLC and Johnson & Johnson stockholders.
Key words: Paliperidone, schizoaffective disorder, depressive symptoms, path analysis, direct effects
Abstract
Background. This analysis evaluates improvement in symptoms of depression in patients with schizoaffective disorder administered oral paliperidone extended-release by accounting for the magnitude of direct and indirect (changes in negative and positive symptoms and worsening of extrapyramidal symptoms) treatment effects on depressive symptoms.
Methods. Data for this post hoc analysis were drawn from two six-week, randomized, placebo-controlled studies of paliperidone extended-release versus placebo in adult subjects with schizoaffective disorder (N=614; NCT00412373, NCT00397033). Subjects with baseline 17-item Hamilton Rating Scale for Depression scores of 16 or greater were included. Structural equation models (path analyses) were used to separate total effects into direct and indirect effects on depressive symptoms. Change from baseline in 17-item Hamilton Rating Scale for Depression score at the Week 6 end point was the dependent variable; changes in Positive and Negative Syndrome Scale positive and negative factors and Simpson-Angus Scale (to evaluate extrapyramidal symptoms) scores were independent variables.
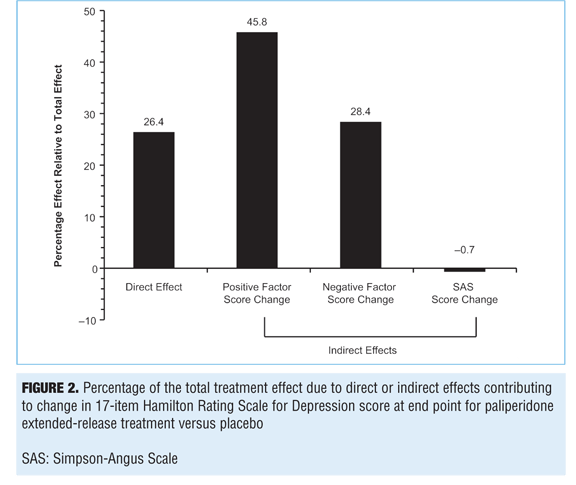
Results: At baseline, 332 of 614 (54.1%) subjects had a 17-item Hamilton Rating Scale for Depression score of 16 or greater. Path analysis determined that up to 26.4 percent of the paliperidone extended-release versus placebo effect on depressive symptoms may be attributed to a direct treatment effect, and 45.8 percent and 28.4 percent were mediated indirectly through improvements on positive and negative symptoms, respectively. No effects were identified as mediated through extrapyramidal symptoms
changes (-0.7%).
Conclusion: Results of this analysis suggest that paliperidone’s effect on depressive symptoms in subjects with schizoaffective disorder participating in two six-week, randomized, placebo-controlled studies is mediated through indirect effects (e.g., positive and negative symptom changes) and a direct treatment effect.
Introduction
Patients with schizoaffective disorder (SCA) often experience complex symptomatology that is characterized by psychotic, depressive, and manic symptoms.[1–3] To manage these diverse symptom groupings, healthcare providers (HCPs) often use mood stabilizers and/or antidepressants in combination with antipsychotics.[4–6] Paliperidone extended-release (ER) (9-OH-risperidone extended-release) is an antipsychotic that has been shown to reduce psychotic symptoms as well as depressive and manic mood symptoms in SCA.[7–9]
When trying to understand the multifaceted treatment response observed following the use of paliperidone in persons with SCA, HCPs may find it difficult to determine whether the observed improvements in a given symptom domain have been achieved through direct actions on the symptom domain in question or are achieved indirectly through effects on other symptom domains. Resolving the different types of effects due to treatment and knowing the completeness of the direct response on a symptom grouping, like those associated with depression, is important for the development of an overall treatment plan. If a treatment does not have a direct effect on a symptom grouping, its use as monotherapy will likely be insufficient to manage those symptoms. Thus, to manage depressive symptoms in persons with SCA, it is important to understand a drug’s potential direct effects to help determine whether and/or when to add adjunctive antidepressant and/or mood-stabilizing medication to a backbone of treatment with paliperidone. If monotherapy has a significant direct effect on a symptom grouping, the benefit of adding adjunctive therapy must be weighed against the potential for additional risk due to polypharmacy.
Negative and depressive symptoms observed in persons with schizophrenia or SCA can be difficult to tell apart. Although factor analyses indicate that symptoms such as loss of drive/interest (negative symptoms), positive symptoms (especially responses to paranoia), extrapyramidal symptoms (EPS), and loss of energy (associated with depression) are distinguishable and have different functional bases, they have considerable phenotypic overlap and may be difficult to separate during clinical evaluations.[10–14] Correlations observed between variables, such as in treatment response for negative symptoms and depressive symptoms, may not be indicative of a common etiological relationship because observed correlations may reflect many noncausal influences. Path analytic methodological approaches[15] can be employed to separate the total treatment effect for a symptom domain into direct and indirect effects,[16–18] and can specify causal linkages between variables.
Path analysis was used in the current study to examine the direct and indirect effects of paliperidone ER treatment on depressive symptoms in persons with SCA. For this analysis, data were drawn from two randomized, placebo-controlled efficacy studies[7,8] from which treatment responses of positive symptoms, negative symptoms, and EPS were examined as possible indirect mediators of depressive symptom changes. In addition, the treatment response of paliperidone ER on depressive symptoms and the impact of potential moderators were examined. Mediator variables specify how or why a particular effect or relationship occurs and help describe the psychological processes that drive the relationship. A moderator variable represents an effect that changes the strength of an effect or relationship between two variables; it provides an indicator of when or under what conditions a particular effect can be expected. A moderator can be a qualitative (e.g., gender, race, treatment class) or quantitative (e.g., level of baseline 17-item Hamilton Rating Scale for Depression [HAM-D-17] score) variable that affects the direction and/or strength of the relationship between an independent and dependent variable.
Methods
Study design. This post hoc analysis used a pooled database (N=614) composed of two six-week, randomized, placebo-controlled studies of paliperidone ER treatment versus placebo in subjects with SCA (NCT00412373, NCT00397033).[7,8] These two studies had similar subject populations and study designs. Key inclusion criteria included a confirmed diagnosis for SCA (using the Structured Clinical Interview for DSM-IV); Young Mania Rating Scale[19] score of 16 or greater and/or a 21-item Hamilton Rating Scale for Depression (HAM-D-21)[20] score of 16 or greater; a Positive and Negative Syndrome Scale (PANSS)[21] total score of 60 or greater; and a score of 4 or greater on at least two PANSS items (i.e., hostility, excitement, tension, uncooperativeness, and poor impulse control). Subjects were allowed to continue treatment with antidepressants and/or mood stabilizers if the dosages had been stable during the 30 days prior to screen.
Analysis. This post hoc analysis focused on subjects with prominent depressive symptoms, defined as a HAM-D-17 total score of 16 or greater at baseline (N=332). Structural equation models (path analysis) separated the total effects of paliperidone ER treatment into direct and indirect effects on depressive symptoms. Path coefficients determined the direct and indirect effects of paliperidone ER treatment on depressive symptoms versus placebo. The dependent variable was the change in HAM-D-17 score at the Week 6 end point; independent variables were the change in PANSS positive and negative factor scores[22] and the change in Simpson-Angus Scale (SAS) score[23] for EPS at the Week 6 end point. These variables were considered as potential mediators of change in depressive scores based on clinical relationships suggested by the literature.[16,17,24] The “direct treatment effect” was defined as the residual effect remaining after controlling for improvements in PANSS positive and negative symptoms and the presence of EPS. In each regression equation, a factor for treatment was also included so that comparisons between paliperidone ER treatment and placebo could be made. Partial correlations between positive and negative symptoms were accounted for in modeling by including the correlation of their error term variances. No adjustments were made for multiplicity.
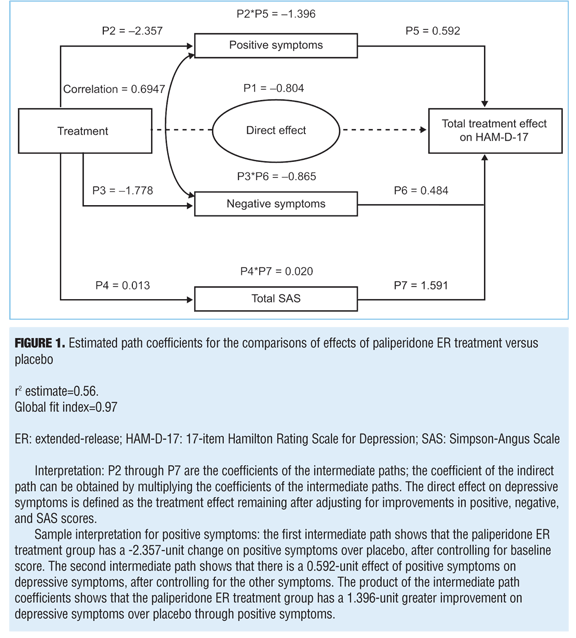
The system of regression models is shown in Table 1. In these equations, baseline in each formula indicates the baseline score of the represented variable. The total effect of treatment has been determined by P1 + (P2 * P5) + (P3 * P6) + (P4 * P7). The variable P1 is the direct effect of treatment, and P2 through P7 are the coefficients of the intermediate paths; the coefficient of the indirect path can be obtained by multiplying the coefficients of the intermediate paths.
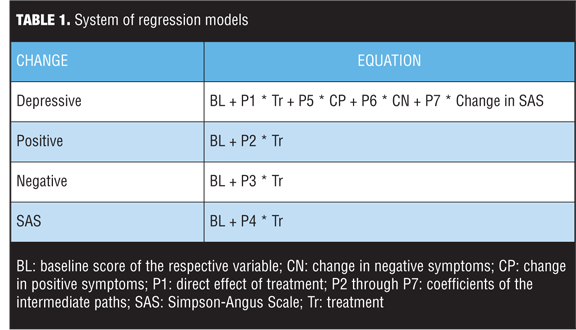
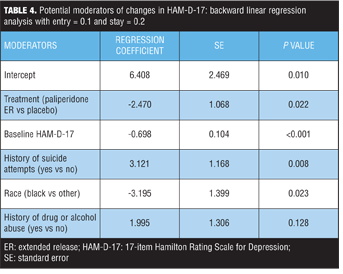
Potential baseline moderators of change in HAM-D-17, such as age, race, gender, duration of current episode, duration of illness, history of suicide attempts, number of previous hospitalizations, substance abuse history, concomitant medication strata (antidepressants and/or mood stabilizers), and schizoaffective subtype (bipolar and depressive), were examined using multiple linear regression models with backward elimination. The analysis started with a complex model, and terms were removed sequentially.
Differences between paliperidone ER and placebo treatment groups in baseline demographics and clinical characteristics were evaluated using analysis of variance models for continuous variables and Fisher’s exact test for categorical variables. Differences between paliperidone ER treatment and placebo on change scores were evaluated using analysis of covariance models with fixed effects for treatment, concomitant medication strata (with or without adjunctive antidepressants and/or mood stabilizers), study identification (ID), country nested within study ID, and baseline score as a covariate. Last observation carried forward methodology was used.
Results
Baseline demographics and clinical characteristics. A total of 332 subjects treated with paliperidone ER or placebo who had HAM-D-17 scores of 16 or greater were identified. No significant differences in baseline demographics and clinical characteristics were observed between treatment groups (Table 2).
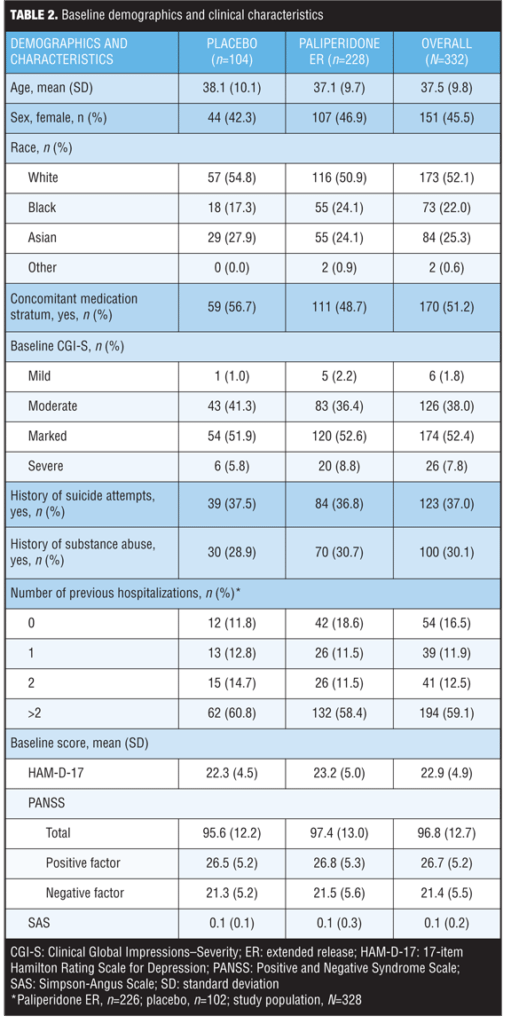
Changes in clinical scales. Compared to placebo, paliperidone ER was associated with significant improvements in mean changes in psychotic and depression symptom scores at Week 6 for all assessments on psychotic and depression symptoms evaluated (P<0.05) (Table 3). SAS scores were low at baseline and end point in both groups.
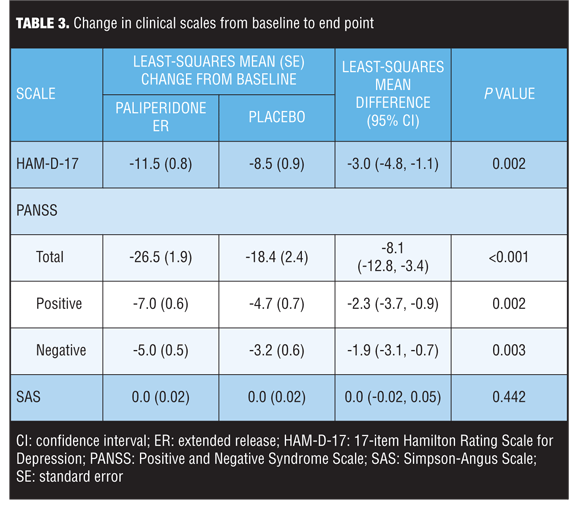
Path analysis. A path analysis was performed using HAM-D-17 scores at Week 6 as a dependent variable; PANSS positive and negative factors and change in SAS scores (for EPS) at Week 6 were used as independent variables (mediators). Results indicate that the treatment effect corresponds to a 3.045-point greater improvement in the HAM-D-17 score in the paliperidone ER group than in the placebo group (P=0.002) (Figure 1). Of the overall depressive symptom improvement, 45.8 percent could be classified as indirectly related to improvements in positive symptoms and 28.4 percent to improvements in negative symptoms. Up to 26.4 percent of treatment effect was identified as a direct effect of paliperidone on depressive symptoms, but no effects were identified as being mediated through EPS changes (-0.7%) (Figures 1 and 2).
Potential moderators of changes in HAM-D-17 score. The following potential baseline moderators were examined: age, race, sex, duration of current episode, duration of illness, history of suicide attempts, number of previous hospitalizations, substance abuse history, co-medication strata (antidepressants and/or mood stabilizers), schizoaffective subtype, and symptom scores. Additionally, the multiple regression model included both treatment and baseline HAM-D-17 scores as explanatory variables. Three demographic variables emerged as potential moderators of change in depressive symptoms: history of suicide attempts, race, and history of substance use (Table 4). In addition to treatment and baseline HAM-D-17 scores, a history of suicide attempts was one of the most significant (P=0.008) treatment moderators of changes in HAM-D-17 score. The magnitude of the regression coefficients depends on the scales of measurement used for the dependent variable and the explanatory variables included in the regression equation. Nonstandardized regression coefficients cannot be compared directly because of differing units of measurements and different variances of the explanatory variables. On average, subjects with a history of suicide attempts had 3.121 units less reduction in HAM-D-17 scores regardless of treatment arms, compared to those subjects without a history of suicide attempts while holding all other variables constant.
Discussion
The aim of this analysis was to examine the direct and indirect effects of paliperidone treatment on depressive symptoms using path analysis methodology in persons with SCA with prominent depressive and psychotic symptoms.
The HAM-D-17, which is widely used to assess mood symptoms in clinical trials, was used to measure depression.[20,25] In the current analysis, paliperidone ER demonstrated an 11.5-unit reduction from baseline in the HAM-D-17 score and a significantly greater improvement compared with placebo (least-squares mean difference: -3.0; 95% confidence interval [CI]: -4.8, -1.1; P=0.002). A 3-point difference in HAM-D-17 score is close to the 3.6-point difference in HAM-D-17 score determined by Turkoz et al corresponding to a one-category change in the Clinical Global Impression of Severity Scale for Schizoaffective Disorder,[26] suggesting that the treatment response with paliperidone ER was clinically meaningful.
The degree of correlation between depression and negative symptoms among patients is unclear. Some studies have reported a meaningful correlation between depression and negative symptoms,[13] while others have not.[11,16,27] For example, one study identified a significant correlation between negative symptoms and depression in female but not male subjects.[28] It has been suggested that negative symptoms and depression have distinct neuroanatomical bases.[11] Depressive symptoms may involve the ventromedial prefrontal cortex and the dorsolateral prefrontal cortex, whereas negative symptoms are more likely to involve the medial frontal lobe,[29] anterior cingulate,[30,31] and medial temporal lobe.[32] Negative symptoms may be associated with neurodevelopmental impairment and have, therefore, been associated with poor outcomes.[27] Although there may be overlap, it is possible to differentiate between depression and negative symptoms by careful clinical evaluation.[13,14,33]
Limitations. The application of a path analytic method to the studies selected for this analysis is limited because the studies were not designed to specifically assess direct or indirect treatment effects on depressive symptoms. As such, the chosen patient populations may not have been fully representative of the symptoms of interest or representative of a patient’s responsiveness to treatment with paliperidone ER. In addition, the assessment parameters evaluated in these studies may not have optimally captured depression, negative symptoms, positive symptoms, or EPS. For example, the Marder negative symptom factor of the PANSS does not capture key elements of the negative symptom construct, such as lack of motivation or diminished emotion and affect. Similarly, although the SAS is widely used to assess parkinsonism, it does not provide a comprehensive assessment of parkinsonian symptoms and some items are vaguely described.[23] The SAS does, however, focus on rigidity, which may represent much of the phenotypic overlap with depression. Another difficulty with the current analysis is that SAS scores were low at baseline and end point in both treatment groups; this resulted in a “floor effect” that made it difficult to demonstrate potential mediating effects. Consequently, persons with higher levels of parkinsonism, who are not represented in this study population, may have different mediation relationships for depression than those described here. In addition, although the 0duration for both studies (6 weeks) was standard for assessing early treatment response, the results could differ from those reported in studies with different durations.
Path analyses are particularly sensitive to model specification; failure to include relevant causal variables or the inclusion of extraneous variables can substantially affect results. The r2 estimate of the initial path analysis was 0.56; this indicates that 56 percent of the total variation of change in depressive symptoms is explained by the mediating variables, including treatment. Because these two factor scores are significantly correlated, the model parameter estimates for PANSS positive and negative scores may be inappropriate and/or the variances of these estimates may be inflated. Path analyses can only consider the secondary effects of factors included in the model. Although the factors identified for inclusion in the present analysis are considered major contributors to depressive symptoms in SCA, other unidentified factors may be important contributors. Further analyses of additional studies are needed to consider these limitations.
Conclusion
These results suggest that paliperidone ER treatment has a direct treatment effect on depressive symptoms (26.4%) in subjects with SCA and prominent symptoms of depression (HAM-D-17 score of ?16). Indirect effects mediated through positive and negative symptom changes (45.8% and 28.4%, respectively) were also identified.
Acknowledgments
The authors thank Sheena Hunt, PhD; Maxwell Chang, BSc Hons; and Matthew Grzywacz, PhD, for providing writing and editorial assistance with this article.
This study was previously presented at the 7th Annual International Society for CNS Clinical Trials and Methodology Autumn Conference, October 3–4, 2011, Amelia Island, Florida, USA.
All authors of this study were directly involved in the design of the analysis; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit this paper for publication. Fu, Bossie, and Alphs were responsible for the design, data collection, and writing. Turkoz was responsible for the design, data collection, writing, and statistical analyses. All authors critically reviewed and revised the manuscript and approved the final manuscript.
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
2. Malaspina D, Owen MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150:21–25.
3. Pagel T, Franklin J, Baethge C. Schizoaffective disorder diagnosed according to different diagnostic criteria–systematic literature search and meta-analysis of key clinical characteristics and heterogeneity. J Affect Disorders. 2014;156:111–118.
4. Keck PE Jr, McElroy SL, Strakowski SM. Schizoaffective disorder: role of atypical antipsychotics. Schizophr Res. 1999;35(Suppl):S5–S12.
5. Lasser R, Bossie CA, Gharabawi G, et al. Efficacy and safety of long-acting risperidone in stable patients with schizoaffective disorder. J Affect Disorders. 2004;83:263–275.
6. Olfson M, Marcus SC, Wan GJ. Treatment patterns for schizoaffective disorder and schizophrenia among Medicaid patients. Psychiatr Serv. 2009;60:210–216.
7. Canuso CM, Lindenmayer JP, Kosik-Gonzalez C, et al. A randomized, double-blind, placebo-controlled study of 2 dose ranges of paliperidone extended-release in the treatment of subjects with schizoaffective disorder. J Clin Psychiatry. 2010;71:587–598.
8. Canuso CM, Schooler N, Carothers J, et al. Paliperidone extended-release in schizoaffective disorder: a randomized, controlled study comparing a flexible dose with placebo in patients treated with and without antidepressants and/or mood stabilizers. J Clin Psychopharmacol. 2010;30:487–495.
9. Canuso CM, Turkoz I, Fu DJ, Bossie CA. Role of paliperidone extended-release in treatment of schizoaffective disorder. Neuropsychiatr Dis Treat. 2010;6:667–679.
10. Fitzgerald PB, Rolfe TJ, Brewer K, et al. Depressive, positive, negative and parkinsonian symptoms in schizophrenia. Aust N Z J Psychiatry. 2002;36:340–346.
11. Kulhara P, Avasthi A, Chadda R, et al. Negative and depressive symptoms in schizophrenia. Br J Psychiatry. 1989;154:207–211.
12. Muller MJ, Szegedi A, Wetzel H, Benkert O. Depressive factors and their relationships with other symptom domains in schizophrenia, schizoaffective disorder, and psychotic depression. Schizophr Bull. 2001;27:19–28.
13. Newcomer JW, Faustman WO, Yeh W, Csernansky JG. Distinguishing depression and negative symptoms in unmedicated patients with schizophrenia. Psychiatry Res. 1990;31:243–250.
14. Whiteford HA, Riney SJ, Csernansky JG. Distinguishing depressive and negative symptoms in chronic schizophrenia. Psychopathol. 1987;20:234–236.
15. Pedhazur EJ. Multiple Regression in Behavioral Research. New York, NY: Holt; 1982.
16. Moller HJ, Muller H, Borison RL, et al. A path-analytical approach to differentiate between direct and indirect drug effects on negative symptoms in schizophrenic patients. A re-evaluation of the North American risperidone study. Eur Arch Psychiatry Clin Neurosci. 1995;245:45–49.
17. Tollefson GD, Sanger TM. Negative symptoms: a path analytic approach to a double-blind, placebo- and haloperidol-controlled clinical trial with olanzapine. Am J Psychiatry. 1997;154:466–474.
18. Turkoz I, Bossie CA, Dirks B, Canuso CM. Direct and indirect effects of paliperidone extended-release tablets on negative symptoms of schizophrenia. Neuropsychiatr Dis Treat. 2008;4:949–958.
19. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435.
20. Hamilton M. Development of a rating scale for primary depressive illness. Br J Social Clin Psychol. 1967;6:278–296.
21. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276.
22. Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58:538–546.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;212:11–19.
24. Tandon R. Quetiapine has a direct effect on the negative symptoms of schizophrenia. Hum Psychopharmacol. 2004;19:559–563.
25. Furukawa TA, Akechi T, Azuma H, et al. Evidence-based guidelines for interpretation of the Hamilton Rating Scale for Depression. J Clin Psychopharmacol. 2007;27:531–534.
26. Turkoz I, Fu DJ, Bossie CA, et al. Relationship between the clinical global impression of severity for schizoaffective disorder scale and established mood scales for mania and depression. J Affect Disorders. 2013;150:17–22.
27. Oosthuizen P, Emsley RA, Roberts MC, et al. Depressive symptoms at baseline predict fewer negative symptoms at follow-up in patients with first-episode schizophrenia. Schizophr Res. 2002;58:247–252.
28. Muller MJ. Gender-specific associations of depression with positive and negative symptoms in acute schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1095–1100.
29. Ho BC, Andreasen NC, Nopoulos P, et al. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594.
30. Egerton A, Brugger S, Raffin M, et al. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacol. 2012;37:2515–2521.
31. Yan H, Tian L, Yan J, et al. Functional and anatomical connectivity abnormalities in cognitive division of anterior cingulate cortex in schizophrenia. PLoS ONE. 2012;7:e45659.
32. Benoit A, Bodnar M, Malla AK, et al. The structural neural substrates of persistent negative symptoms in first-episode of non-affective psychosis: a voxel-based morphometry study. Front Psychiatry. 2012;3:42.
33. Perenyi A, Norman T, Hopwood M, Burrows G. Negative symptoms, depression, and parkinsonian symptoms in chronic, hospitalised schizophrenic patients. J Affect Disorders. 1998;48:163–169.





